Magnetic resonance assessment of the cerebral alterations associated with obesity development
- PMID: 32703110
- PMCID: PMC7585928
- DOI: 10.1177/0271678X20941263
Magnetic resonance assessment of the cerebral alterations associated with obesity development
Abstract
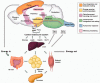
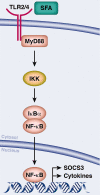
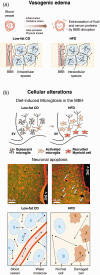
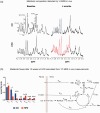
Obesity is a current threat to health care systems, affecting approximately 13% of the world's adult population, and over 18% children and adolescents. The rise of obesity is fuelled by inadequate life style habits, as consumption of diets rich in fats and sugars which promote, additionally, the development of associated comorbidities. Obesity results from a neuroendocrine imbalance in the cerebral mechanisms controlling food intake and energy expenditure, including the hypothalamus and the reward and motivational centres. Specifically, high-fat diets are known to trigger an early inflammatory response in the hypothalamus that precedes weight gain, is time-dependent, and eventually extends to the remaining appetite regulating regions in the brain. Multiple magnetic resonance imaging (MRI) and spectroscopy (MRS) methods are currently available to characterize different features of cerebral obesity, including diffusion weighted, T2 and volumetric imaging and 1H and 13C spectroscopic evaluations. In particular, consistent evidences have revealed increased water diffusivity and T2 values, decreased grey matter volumes, and altered metabolic profiles and fluxes, in the brain of animal models and in obese humans. This review provides an integrative interpretation of the physio-pathological processes associated with obesity development in the brain, and the MRI and MRS methods implemented to characterize them.
Keywords: High-fat feeding; hypothalamus; magnetic resonance imaging; magnetic resonance spectroscopy; neuro-inflammation; obesity.
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures

References
-
- Apovian CM.Obesity: definition, comorbidities, causes, and burden. Am J Manag Care 2016; 22: s176–185. - PubMed
-
- Frisardi V, Solfrizzi V, Seripa D, et al.. Metabolic-cognitive syndrome: a cross-talk between metabolic syndrome and Alzheimer's disease. Age Res Rev 2010; 9: 399–417. - PubMed
-
- Smith KB, Smith MS.Obesity statistics. Prim Care 2016; 43: 121–135, ix. - PubMed
-
- Flegal KM, Carroll MD, Ogden CL, et al.. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010; 303: 235–241. - PubMed

