Three-dimensional morphometric analysis reveals time-dependent structural changes in microglia and astrocytes in the central amygdala and hypothalamic paraventricular nucleus of heart failure rats
- PMID: 32703230
- PMCID: PMC7379770
- DOI: 10.1186/s12974-020-01892-4
Three-dimensional morphometric analysis reveals time-dependent structural changes in microglia and astrocytes in the central amygdala and hypothalamic paraventricular nucleus of heart failure rats
Erratum in
-
Correction to: Three-dimensional morphometric analysisreveals time-dependent structural changesin microglia and astrocytes in the centralamygdala and hypothalamicparaventricular nucleus of heart failure rats.J Neuroinflammation. 2020 Nov 22;17(1):348. doi: 10.1186/s12974-020-02035-5. J Neuroinflammation. 2020. PMID: 33222695 Free PMC article.
Abstract
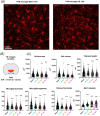
Background: Cardiovascular diseases, including heart failure, are the most common cause of death globally. Recent studies support a high degree of comorbidity between heart failure and cognitive and mood disorders resulting in memory loss, depression, and anxiety. While neuroinflammation in the hypothalamic paraventricular nucleus contributes to autonomic and cardiovascular dysregulation in heart failure, mechanisms underlying cognitive and mood disorders in this disease remain elusive. The goal of this study was to quantitatively assess markers of neuroinflammation (glial morphology, cytokines, and A1 astrocyte markers) in the central amygdala, a critical forebrain region involved in emotion and cognition, and to determine its time course and correlation to disease severity during the progression of heart failure.
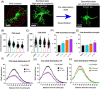
Methods: We developed and implemented a comprehensive microglial/astrocyte profiler for precise three-dimensional morphometric analysis of individual microglia and astrocytes in specific brain nuclei at different time points during the progression of heart failure. To this end, we used a well-established ischemic heart failure rat model. Morphometric studies were complemented with quantification of various pro-inflammatory cytokines and A1/A2 astrocyte markers via qPCR.
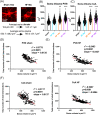
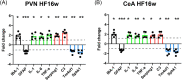
Results: We report structural remodeling of central amygdala microglia and astrocytes during heart failure that affected cell volume, surface area, filament length, and glial branches, resulting overall in somatic swelling and deramification, indicative of a change in glial state. These changes occurred in a time-dependent manner, correlated with the severity of heart failure, and were delayed compared to changes in the hypothalamic paraventricular nucleus. Morphometric changes correlated with elevated mRNA levels of pro-inflammatory cytokines and markers of reactive A1-type astrocytes in the paraventricular nucleus and central amygdala during heart failure.
Conclusion: We provide evidence that in addition to the previously described hypothalamic neuroinflammation implicated in sympathohumoral activation during heart failure, microglia, and astrocytes within the central amygdala also undergo structural remodeling indicative of glial shifts towards pro-inflammatory phenotypes. Thus, our studies suggest that neuroinflammation in the amygdala stands as a novel pathophysiological mechanism and potential therapeutic target that could be associated with emotional and cognitive deficits commonly observed at later stages during the course of heart failure.
Keywords: A1; Amygdala; Astrocytes; Autonomic; Behavior; Cytokines; Heart failure; Hypothalamus; Microglia; Neuroinflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Beemath A, Stein PD, Skaf E, Al Sibae MR, Alesh I. Risk of venous thromboembolism in patients hospitalized with heart failure. Am J Cardiol. 2006;98(6):793–795. - PubMed
-
- Parissis JT, Fountoulaki K, Paraskevaidis I, Kremastinos D. Depression in chronic heart failure: novel pathophysiological mechanisms and therapeutic approaches. Expert Opin Investig Drugs. 2005;14(5):567–577. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

