Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.)
- PMID: 32703232
- PMCID: PMC7376921
- DOI: 10.1186/s12951-020-00659-6
Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.)
Abstract
Background: Selenium (Se) in soil mainly consists of selenite, selenate, and elemental Se. However, little is known about the mechanism involved in the uptake and biotransformation of elemental Se by plants.
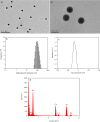
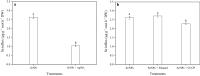
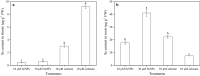
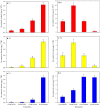
Results: In this study, the uptake, translocation, subcellular distribution and biotransformation of selenium nanoparticles (SeNPs) in rice (Oryza sativa L.), and a comparison with selenite and selenate, were investigated through hydroponic experiments. The study revealed that SeNPs could be absorbed by rice plants; and aquaporin inhibitor was responsible for a 60.4% inhibition of SeNP influx, while metabolic inhibitor was ineffective. However, the SeNPs uptake rate of rice roots was approximately 1.7 times slower than that of selenite or selenate. Under the SeNPs or selenite treatment, Se was primarily accumulated in roots rather than in shoots, whereas an opposite trend was observed with selenate treatment. Additionally, most of the absorbed Se was distributed in cell wall of the SeNPs or selenite treated-rice plants, while its proportion was the highest in soluble cytosol of the selenate treated-rice plants. The absorbed SeNPs or selenite was rapidly assimilated to organic forms, with SeMet being the most predominant species in both shoots and roots of the rice plants. However, following selenate treatment, Se(VI) remained as the most predominant species, and only a small amount of it was converted to organic forms.
Conclusion: Therefore, this study provides a deeper understanding of the mechanisms associated SeNPs uptake and biotransformation within plants.
Keywords: Assimilation; Inorganic Se; Plants; Se nanoparticles; Subcellular distribution; Uptake.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Rayman MP. Selenium and human health. Lancet. 2012;379(9822):1256–1268. - PubMed
-
- Fairweather-Tait SJ, Bao Y, Broadley MR, Collings R, Ford D, Hesketh JE, et al. Selenium in human health and disease. Antioxid Redox Sign. 2011;14(7):1337–1383. - PubMed
-
- Rayman MP. The importance of selenium to human health. Lancet. 2000;356(9225):233–241. - PubMed
-
- Lv Y, Yu T, Yang Z, Zhao W, Zhang M, Wang Q. Constraint on selenium bioavailability caused by its geochemical behavior in typical Kaschin-Beck disease areas in Aba, Sichuan Province of China. Sci Total Environ. 2014;493:737–749. - PubMed
-
- Combs GF. Selenium in global food systems. Brit J Nutr. 2001;85(5):517–547. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

