Azidophosphonium salt-directed chemoselective synthesis of (E)/(Z)-cinnamyl-1 H-triazoles and regiospecific access to bromomethylcoumarins from Morita-Baylis-Hillman adducts
- PMID: 32704324
- PMCID: PMC7356223
- DOI: 10.3762/bjoc.16.130
Azidophosphonium salt-directed chemoselective synthesis of (E)/(Z)-cinnamyl-1 H-triazoles and regiospecific access to bromomethylcoumarins from Morita-Baylis-Hillman adducts
Abstract
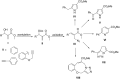
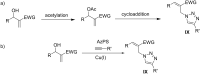
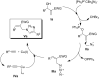
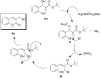
The direct transformation of Morita-Baylis-Hillman (MBH) adducts into molecules of interest is a crucial process wherein allylic hydroxy-protected or halogenated MBH adducts are commonly preferred. Herein, we report an azidophosphonium salt (AzPS)-catalysed straight forward protocol for synthesising structurally demanding (E)/(Z)-cinnamyl-1H-1,2,3-triazoles and halomethylcoumarins from MBH adducts. The novel methodology, efficient catalyst, and direct utilization of MBH adducts under mild reaction conditions qualify the reported procedures as powerful synthetic tools.
Keywords: Morita–Baylis–Hillman adducts; halomethylcoumarin; organocatalyst; phosphonium salt; triazolation.
Copyright © 2020, Karthikeyan et al.; licensee Beilstein-Institut.
Figures


Similar articles
-
Transformations of Modified Morita-Baylis-Hillman Adducts from Isatins Catalyzed by Lewis Bases.Chem Rec. 2020 Jun;20(6):541-555. doi: 10.1002/tcr.201900058. Epub 2019 Oct 14. Chem Rec. 2020. PMID: 31609533 Review.
-
The Application of Biocatalysis in the Preparation and Resolution of Morita-Baylis-Hillman Adducts and Their Derivatives.Chembiochem. 2022 Apr 5;23(7):e202100527. doi: 10.1002/cbic.202100527. Epub 2021 Dec 6. Chembiochem. 2022. PMID: 34822736 Review.
-
Synthesis, biological activities, and structure-activity relationships of Morita-Baylis-Hillman adducts: An update.Arch Pharm (Weinheim). 2024 Oct;357(10):e2400372. doi: 10.1002/ardp.202400372. Epub 2024 Jul 4. Arch Pharm (Weinheim). 2024. PMID: 38963326 Review.
-
Lipase-Catalysed Enzymatic Kinetic Resolution of Aromatic Morita-Baylis-Hillman Derivatives by Hydrolysis and Transesterification.Chembiochem. 2022 Nov 4;23(21):e202200435. doi: 10.1002/cbic.202200435. Epub 2022 Sep 29. Chembiochem. 2022. PMID: 36049111 Free PMC article.
-
Organocatalyzed Decarboxylative Trichloromethylation of Morita-Baylis-Hillman Adducts in Batch and Continuous Flow.Chemistry. 2018 Jan 24;24(5):1204-1208. doi: 10.1002/chem.201704972. Epub 2017 Dec 15. Chemistry. 2018. PMID: 29168579
References
-
- Mamaghani M, Badrian A. Tetrahedron Lett. 2004;45:1547–1550. doi: 10.1016/j.tetlet.2003.12.046. - DOI
-
- Yadav L D S, Srivastava V P, Patel R. Tetrahedron Lett. 2008;49:3142–3146. doi: 10.1016/j.tetlet.2008.03.016. - DOI
-
- Tu S, Xu L-H, Yu C-R. Synth Commun. 2008;38:2662–2671. doi: 10.1080/00397910601038731. - DOI
-
- Gowrisankar S, Kim K-H, Kim J-N. Bull Korean Chem Soc. 2008;29:2537–2539. doi: 10.5012/bkcs.2008.29.12.2537. - DOI
LinkOut - more resources
Full Text Sources
