Transcutaneous bilirubinometry during and after phototherapy in preterm infants: a prospective observational study
- PMID: 32704577
- PMCID: PMC7368502
- DOI: 10.1136/bmjpo-2020-000681
Transcutaneous bilirubinometry during and after phototherapy in preterm infants: a prospective observational study
Abstract
Objective: To examine the accuracy of transcutaneous bilirubinometry (TCB) measurements during and after phototherapy (PT) in preterm infants.
Design: Prospective observational cohort study.
Setting: Level III neonatal centre.
Patients: Preterm infants (from 23+0 to 36+6 weeks of gestation) born between June 2017 and May 2018 requiring PT.
Interventions: TCB was measured from an exposed area of the skin (the sternum; TCBU) and the covered area of the skin under the nappy (the bony part of the upper outer quadrant of the buttock; TCBC) within an hour of obtaining total serum bilirubin (TSB).
Main outcome measures: Correlation and agreement between TCB (TCBU and TCBC) and TSB during and after PT.
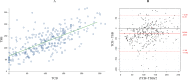
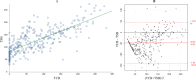
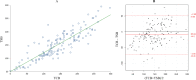
Results: We have enrolled 196 preterm infants. There was a significant correlation between TSB and TCB during PT (r=0.72, 95% CI 0.66 to 0.77 in covered area; r=0.75, 95% CI 0.70 to 0.80 in uncovered area) and after PT (r=0.87, 95% CI 0.83 to 0.91). TCB underestimated TSB level during PT, with a mean TCBC-TSB difference of -25±43 (95% agreement limits of 62 to -112) and a mean TCBU-TSB difference of -48±46 (95% agreement limits of 45 to -140). The agreement between TCB and TSB after cessation of PT improved, with TCB underestimating TSB by a mean TCB-TSB difference of -10±31 (95% agreement limits of 52 to -72).
Conclusion: TCB measurements correlated strongly with TSB levels during and after PT. However, there was a wide and clinically relevant disagreement between TCB and TSB measurements during the PT phase, improving significantly after PT.
Keywords: jaundice; monitoring; neonatology.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
LinkOut - more resources
Full Text Sources
