Impaired glymphatic function and clearance of tau in an Alzheimer's disease model
- PMID: 32705145
- PMCID: PMC7447521
- DOI: 10.1093/brain/awaa179
Impaired glymphatic function and clearance of tau in an Alzheimer's disease model
Abstract
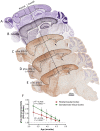
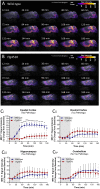
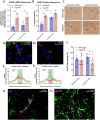
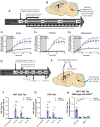
The glymphatic system, that is aquaporin 4 (AQP4) facilitated exchange of CSF with interstitial fluid (ISF), may provide a clearance pathway for protein species such as amyloid-β and tau, which accumulate in the brain in Alzheimer's disease. Further, tau protein transference via the extracellular space, the compartment that is cleared by the glymphatic pathway, allows for its neuron-to-neuron propagation, and the regional progression of tauopathy in the disorder. The glymphatic system therefore represents an exciting new target for Alzheimer's disease. Here we aim to understand the involvement of glymphatic CSF-ISF exchange in tau pathology. First, we demonstrate impaired CSF-ISF exchange and AQP4 polarization in a mouse model of tauopathy, suggesting that this clearance pathway may have the potential to exacerbate or even induce pathogenic accumulation of tau. Subsequently, we establish the central role of AQP4 in the glymphatic clearance of tau from the brain; showing marked impaired glymphatic CSF-ISF exchange and tau protein clearance using the novel AQP4 inhibitor, TGN-020. As such, we show that this system presents as a novel druggable target for the treatment of Alzheimer's disease, and possibly other neurodegenerative diseases alike.
Keywords: Alzheimer’s disease; aquaporin-4; glymphatic; rTg4510; tau.
© The Author(s) (2020). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures


References
-
- Amiry-Moghaddam M, Frydenlund DS, Ottersen OP.. Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 2004; 129: 997–1008. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

