Altered spontaneous brain activity patterns in diabetic patients with vitreous hemorrhage using amplitude of low‑frequency fluctuation: A resting‑state fMRI study
- PMID: 32705185
- PMCID: PMC7411342
- DOI: 10.3892/mmr.2020.11294
Altered spontaneous brain activity patterns in diabetic patients with vitreous hemorrhage using amplitude of low‑frequency fluctuation: A resting‑state fMRI study
Abstract
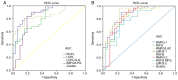
The aim of the present study was to assess the local character of spontaneous brain activity in type‑2 diabetic patients with vitreous hemorrhage (VH) and its relationship with clinical features via the amplitude of low‑frequency fluctuations (ALFF) method. A total of 31 subjects (15 females and 16 males) with type‑2 diabetic VH and 31 normal controls (NCs) with similar characteristics (sex, age and educational level) were recruited in the present study. All subjects underwent resting‑state functional magnetic resonance imaging scans. The local character of spontaneous brain activity was assessed using the ALFF method. The difference between the type‑2 diabetic patients with VH and NCs was determined using receiver operating characteristic curves. Pearson's correlation analysis was applied to evaluate the relationship between the mean ALFF values of specific brain areas and related clinical manifestations in type‑2 diabetic patients with VH. The ALFF values of type‑2 diabetic patients with VH were significantly increased in the right and left cerebellum posterior lobes, left cerebellum posterior lobe/left lingual gyrus and bilateral superior frontal gyrus/left postcentral gyrus, compared with those obtained for NCs (P<0.05). By contrast, these values were significantly decreased in the left and right middle frontal gyri, right medial frontal gyrus/left anterior cingulate, right inferior frontal gyrus, right superior frontal gyrus, right middle frontal gyrus, right superior frontal gyrus/middle frontal gyrus and left middle frontal gyrus of the former group compared with the NCs (P<0.05). Nevertheless, there was no significant association between the mean ALFF values and clinical characteristics in different brain areas. Unusual spontaneous activity occurred in multiple brain areas, which may suggest the neuropathological mechanisms of visual impairment in type‑2 diabetic patients with VH.
Keywords: vitreous hemorrhage; diabetes mellitus; amplitude of low-frequency; spontaneous brain activity.
Figures






References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

