Connective tissue growth factor produced by cancer‑associated fibroblasts correlates with poor prognosis in epithelioid malignant pleural mesothelioma
- PMID: 32705221
- PMCID: PMC7388423
- DOI: 10.3892/or.2020.7669
Connective tissue growth factor produced by cancer‑associated fibroblasts correlates with poor prognosis in epithelioid malignant pleural mesothelioma
Abstract
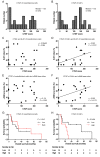
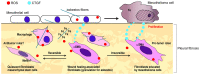
Malignant mesothelioma is an aggressive neoplasm for which effective treatments are lacking. We often encounter mesothelioma cases with a profound desmoplastic reaction, suggesting the involvement of cancer‑associated fibroblasts (CAFs) in mesothelioma progression. While the roles of CAFs have been extensively studied in other tumors and have led to the view that the cancer stroma contains heterogeneous populations of CAFs, their roles in mesothelioma remain unknown. We previously showed that connective tissue growth factor (CTGF), a secreted protein, is produced by both mesothelioma cells and fibroblasts and promotes the invasion of mesothelioma cells in vitro. In this study, we examined the clinical relevance of CAFs in mesothelioma. Using surgical specimens of epithelioid malignant pleural mesothelioma, we evaluated the clinicopathological significance of the expression of α‑smooth muscle actin (αSMA), the most widely used marker of CAFs, the expression of CTGF, and the extent of fibrosis by immunohistochemistry and Elastica‑Masson staining. We also analyzed the expression of mesenchymal stromal cell‑ and fibroblast‑expressing Linx paralogue (Meflin; ISLR), a recently reported CAF marker that labels cancer‑restraining CAFs and differ from αSMA‑positive CAFs, by in situ hybridization. The extent of fibrosis and CTGF expression in mesothelioma cells did not correlate with patient prognosis. However, the expression of αSMA and CTGF, but not Meflin, in CAFs correlated with poor prognosis. The data suggest that CTGF+ CAFs are involved in mesothelioma progression and represent a potential molecular target for mesothelioma therapy.
Keywords: connective tissue growth factor; CTGF; cancer-associated fibroblasts; tumor microenvironment; malignant mesothelioma; mesenchymal stromal cell‑ and fibroblast‑expressing of a Linx paralogue; Meflin; ISLR; molecular target therapy.
Figures



References
-
- International Agency for Research on Cancer, corp-author. A Review of Human Carcinogens; Part C: Arsenic. Metals; Fibres, and Dusts, Lyon, France: 2012. WHO. Asbestos (chrysotile, amosite, crocidolite, tremolite, actinolite, and anthophyllite). IARC Monographs on the Evaluation of Carcinogenic Risks to Humans Volume 100C; pp. 219–309.
-
- Pavlisko EN, Sporn TA. Pathology of asbestos-associated diseases. 3rd. Springer; Berlin: 2014. Mesothelioma; pp. 81–140. - DOI
-
- Donaldson K, Murphy FA, Duffin R, Poland CA. Asbestos, carbon nanotubes and the pleural mesothelium: A review of the hypothesis regarding the role of long fibre retention in the parietal pleura, inflammation and mesothelioma. Part Fibre Toxicol. 2010;7:5. doi: 10.1186/1743-8977-7-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

