New Rheumatoid Arthritis Treatments for 'Old' Patients: Results of a Systematic Review
- PMID: 32705531
- PMCID: PMC7444401
- DOI: 10.1007/s12325-020-01435-6
New Rheumatoid Arthritis Treatments for 'Old' Patients: Results of a Systematic Review
Abstract
Introduction: In the last 20 years, biologic and targeted synthetic disease-modifying antirheumatic drugs (DMARDs) have become available for treating rheumatoid arthritis (RA), and a treat-to-target strategy has been introduced. We hypothesise that these advances should have resulted in changes to the characteristics of patients with RA participating in clinical trials of the newest therapies. This study determined whether the baseline characteristics of patients with RA enrolled in clinical trials have changed in the past decade versus patients participating in earlier RA studies.
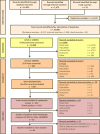
Methods: This secondary analysis was based on randomised controlled trials (RCTs) identified in a systematic literature review. Baseline characteristics of patients with RA with inadequate response to conventional synthetic DMARDs were compared between RCTs published in 1999-2009 and those published in 2010-2017 using random-effects meta-analyses.
Results: Forty RCTs were analysed: 22 from 1999-2009 and 18 from 2010-2017. No significant difference between the two timeframes and no obvious trend over time were observed for age, gender, disease duration, rheumatoid factor status, tender and swollen joint counts, physician and patient global assessments of disease activity, and pain scores. Variability between RCTs was high. Similar results were observed for Disease Activity Scores and Health Assessment Questionnaire-Disability Index scores, but with low variability between RCTs.
Conclusion: The baseline characteristics of patients with RA participating in RCTs do not appear to have changed in the last decade despite the availability of new treatments and a different treatment approach. Further research should determine the impact of baseline patient characteristics on patients' response to RA treatments.
Keywords: Disease-modifying antirheumatic drugs; Patient characteristics; Randomised controlled clinical trials; Rheumatoid arthritis; Rheumatology; Systematic review.
Plain language summary
In the last 20 years, new treatments and a new treatment approach (called treat-to-target) have been introduced for rheumatoid arthritis (RA). Consequently, the characteristics of patients with RA participating in clinical trials of the newest therapies should have changed compared with those of patients who participated in clinical trials of older therapies. This is important as patient characteristics may influence patients’ response to drug treatment. To determine whether characteristics of patients with RA have changed over time, we compared the baseline characteristics (e.g. age, gender, disease duration, measures of disease activity, and pain scores) of patients with RA between 22 clinical trials published in 1999–2009 and 18 published in 2010–2017. No significant difference between the two timeframes and no obvious trend over time were observed for any baseline characteristic of patients with RA, including physician and patient measures of disease activity, and patient measures of physical function and pain. Thus, the baseline characteristics of patients with RA participating in clinical trials do not appear to have changed in the last decade despite the introduction of new treatments and the treat-to-target approach. Further research is needed to determine the impact of baseline patient characteristics on patients’ response to RA treatments.
Figures



References
-
- Verma MK, Sobha K. Understanding the major risk factors in the beginning and the progression of rheumatoid arthritis: current scenario and future prospects. Inflamm Res. 2015;64(9):647–659. - PubMed
-
- Ometto F, Fedeli U, Schievano E, Botsios C, Punzi L, Corti MC. Cause-specific mortality in a large population-based cohort of patients with rheumatoid arthritis in Italy. Clin Exp Rheumatol. 2018;36(4):636–642. - PubMed
-
- Tobón GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: rheumatoid arthritis. J Autoimmun. 2010;35(1):10–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

