Loss of capillary pericytes and the blood-brain barrier in white matter in poststroke and vascular dementias and Alzheimer's disease
- PMID: 32705757
- PMCID: PMC8018063
- DOI: 10.1111/bpa.12888
Loss of capillary pericytes and the blood-brain barrier in white matter in poststroke and vascular dementias and Alzheimer's disease
Abstract
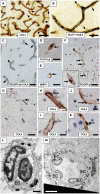
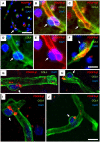
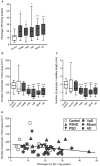
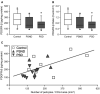
White matter (WM) disease is associated with disruption of the gliovascular unit, which involves breach of the blood-brain barrier (BBB). We quantified pericytes as components of the gliovascular unit and assessed their status in vascular and other common dementias. Immunohistochemical and immunofluorescent methods were developed to assess the distribution and quantification of pericytes connected to the frontal lobe WM capillaries. Pericytes with a nucleus were identified by collagen 4 (COL4) and platelet-derived growth factor receptor-β (PDGFR-β) antibodies with further verification using PDGFR-β-specific ELISA. We evaluated a total of 124 post-mortem brains from subjects with post-stroke dementia (PSD), vascular dementia (VaD), Alzheimer's disease (AD), AD-VaD (Mixed) and post-stroke non-demented (PSND) stroke survivors as well as normal aging controls. COL4 and PDGFR-β reactive pericytes adopted the characteristic "crescent" or nodule-like shapes around capillary walls. We estimated densities of pericyte somata to be 225 ±38 and 200 ±13 (SEM) per COL4 mm2 area or 2.0 ± 0.1 and 1.7 ± 0.1 per mm capillary length in young and older aging controls. Remarkably, WM pericytes were reduced by ~35%-45% in the frontal lobe of PSD, VaD, Mixed and AD subjects compared to PSND and controls subjects (P < 0.001). We also found pericyte numbers were correlated with PDGFR-β reactivity in the WM. Our results first demonstrate a reliable method to quantify COL4-positive pericytes and then, indicate that deep WM pericytes are decreased across different dementias including PSD, VaD, Mixed and AD. Our findings suggest that downregulation of pericytes is associated with the disruption of the BBB in the deep WM in several aging-related dementias.
Keywords: cerebral capillary; collagen type IV; dementia; pericytes; platelet-derived growth factor receptor; vascular dementia; white matter.
© 2020 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors have no disclosures or conflicts of interest in relation to this manuscript.
Figures


References
-
- Armulik A, Genove G, Betsholtz C (2011) Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell 21:193–215. - PubMed
-
- Baradaran H, Mtui EE, Richardson JE, Delgado D, Dunning A, Marshall RS et al (2016) White matter diffusion abnormalities in carotid artery disease: a systematic review and meta‐analysis. J Neuroimaging 26:481–488. - PubMed

