Dynamics of gaze control during prey capture in freely moving mice
- PMID: 32706335
- PMCID: PMC7438109
- DOI: 10.7554/eLife.57458
Dynamics of gaze control during prey capture in freely moving mice
Abstract
Many studies of visual processing are conducted in constrained conditions such as head- and gaze-fixation, and therefore less is known about how animals actively acquire visual information in natural contexts. To determine how mice target their gaze during natural behavior, we measured head and bilateral eye movements in mice performing prey capture, an ethological behavior that engages vision. We found that the majority of eye movements are compensatory for head movements, thereby serving to stabilize the visual scene. During movement, however, periods of stabilization are interspersed with non-compensatory saccades that abruptly shift gaze position. Notably, these saccades do not preferentially target the prey location. Rather, orienting movements are driven by the head, with the eyes following in coordination to sequentially stabilize and recenter the gaze. These findings relate eye movements in the mouse to other species, and provide a foundation for studying active vision during ethological behaviors in the mouse.
Keywords: active sensing; ethology; eye movements; mouse; neuroscience; vision.
Plain language summary
As you read this sentence, your eyes will move automatically from one word to the next, while your head remains still. Moving your eyes enables you to view each word using your central – as opposed to peripheral – vision. Central vision allows you to see objects in fine detail. It relies on a specialized area of the retina called the fovea. When you move your eyes across a page, you keep the images of the words you are currently reading on the fovea. This provides the detailed vision required for reading. The same process works for tracking moving objects. When watching a bird fly across the sky, you can track its progress by moving your eyes to keep the bird in the center of your visual field, over the fovea. But the majority of mammals do not have a fovea, and yet are still able to track moving targets. Think of a lion hunting a gazelle, for instance, or a cat stalking a mouse. Even mice themselves can track and capture insect prey such as crickets, despite not having a fovea. And yet, exactly how they do this is unknown. This is particularly surprising given that mice have long been used to study the neural basis of vision. By fitting mice with miniature head-mounted cameras, Michaiel et al. now reveal how the rodents track and capture moving crickets. It turns out that unlike animals with a fovea, mice do not use eye movements to track moving objects. Instead, when a mouse wants to look at something new, it moves its head to point at the target. The eyes then follow and ‘land’ on the target. In essence, head movements lead the way and the eyes catch up afterwards. These findings are consistent with the idea that mammals with large heads evolved eye movements to overcome the energy costs of turning the head whenever they want to look at something new. For small animals, moving the head is less energetically expensive. As a result, being able to move the eyes independent of the head is unnecessary. Future work could use a combination of behavioral experiments and brain recordings to reveal how visual areas of the brain process what an animal is seeing in real time.
© 2020, Michaiel et al.
Conflict of interest statement
AM, EA, CN No competing interests declared
Figures

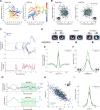
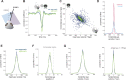


References
-
- Bailey V, Sperry C. Life history and habits of grasshopper mice. [March 25, 2020];Genus Onychomys. 1929 https://ageconsearch.umn.edu/record/157954?ln=en
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

