On the Case of the Misplaced Hydrogens
- PMID: 32706524
- PMCID: PMC7952024
- DOI: 10.1002/cbic.202000376
On the Case of the Misplaced Hydrogens
Abstract
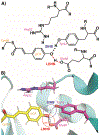
Few other elements play a more central role in biology than hydrogen. The interactions, bonding and movement of hydrogen atoms are central to biological catalysis, structure and function. Yet owing to the elusive nature of a single hydrogen atom few experimental and computational techniques can precisely determine its location. This is exemplified in short hydrogen bonds (SHBs) where the location of the hydrogen atom is indicative of the underlying strength of the bonds, which can vary from 1-5 kcal/mol in canonical hydrogen bonds, to an almost covalent nature in single-well hydrogen bonds. Owing to the often-times inferred position of hydrogen, the role of SHBs in biology has remained highly contested and debated. This has also led to discrepancies in computational, biochemical and structural studies of proteins thought to use SHBs in performing chemistry and stabilizing interactions. Herein, we discuss in detail two distinct examples, namely the conserved catalytic triad and the photoreceptor, photoactive yellow protein, where studies of these SHB-containing systems have permitted contextualization of the role these unique hydrogen bonds play in biology.
Keywords: catalytic triads; hydrogen bonds; low-barrier hydrogen bonds; photoactive yellow proteins; short ionic hydrogen bonds.
© 2020 Wiley-VCH GmbH.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

