Cancer and Leukemia Group B 90203 (Alliance): Radical Prostatectomy With or Without Neoadjuvant Chemohormonal Therapy in Localized, High-Risk Prostate Cancer
- PMID: 32706639
- PMCID: PMC7479762
- DOI: 10.1200/JCO.20.00315
Cancer and Leukemia Group B 90203 (Alliance): Radical Prostatectomy With or Without Neoadjuvant Chemohormonal Therapy in Localized, High-Risk Prostate Cancer
Abstract
Purpose: Radical prostatectomy (RP) alone is often inadequate in curing men with clinically localized, high-risk prostate cancer (PC). We hypothesized that chemohormonal therapy (CHT) with androgen-deprivation therapy plus docetaxel before RP would improve biochemical progression-free survival (BPFS) over RP alone.
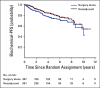
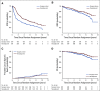
Patients and methods: Men with clinically localized, high-risk PC were assigned to RP alone or neoadjuvant CHT with androgen deprivation plus docetaxel (75 mg/m2 body surface area every 3 weeks for 6 cycles) and RP. The primary end point was 3-year BPFS. Biochemical failure was defined as a serum prostate-specific antigen level > 0.2 ng/mL that increased on 2 consecutive occasions that were at least 3 months apart. Secondary end points included 5-year BPFS, overall BPFS, local recurrence, metastasis-free survival (MFS), PC-specific mortality, and overall survival (OS).
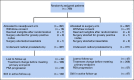
Results: In total, 788 men were randomly assigned. Median follow-up time was 6.1 years. The overall rates of grade 3 and 4 adverse events during chemotherapy were 26% and 19%, respectively. No difference was seen in 3-year BPFS between neoadjuvant CHT plus RP and RP alone (0.89 v 0.84, respectively; 95% CI for the difference, -0.01 to 0.11; P = .11). Neoadjuvant CHT was associated with improved overall BPFS (hazard ratio [HR], 0.69; 95% CI, 0.48 to 0.99), improved MFS (HR, 0.70; 95% CI, 0.51 to 0.95), and improved OS (HR, 0.61; 95% CI, 0.40 to 0.94) compared with RP alone.
Conclusion: The primary study end point, 3-year BPFS, was not met. Although some improvement was seen in secondary end points, any potential benefit must be weighed against toxicity. Our data do not support the routine use of neoadjuvant CHT and RP in patients with clinically localized, high-risk PC at this time.
Trial registration: ClinicalTrials.gov NCT00430183.
Conflict of interest statement
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Figures
References
-
- DeVita VT Jr, Lawrence TS, Rosenberg SA (eds): Cancer of the prostate, in Cancer: Principles and Practice of Oncology (ed 11). Philadelphia, PA, Wolters Kluwer, 2019, 1087.
-
- Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. - PubMed
-
- Klotz LH, Goldenberg SL, Jewett M, et al. CUOG randomized trial of neoadjuvant androgen ablation before radical prostatectomy: 36-month post-treatment PSA results. Urology. 1999;53:757–763. - PubMed
-
- Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. J Urol. 2002;167:112–116. - PubMed
-
- Witjes WP, Schulman CC, Debruyne FM. Preliminary results of a prospective randomized study comparing radical prostatectomy versus radical prostatectomy associated with neoadjuvant hormonal combination therapy in T2-3 N0 M0 prostatic carcinoma. Urology. 1997;49(suppl)( 3A):65–69. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- U10 CA077202/CA/NCI NIH HHS/United States
- U10 CA180821/CA/NCI NIH HHS/United States
- P30 CA008748/CA/NCI NIH HHS/United States
- U10 CA180882/CA/NCI NIH HHS/United States
- UG1 CA233327/CA/NCI NIH HHS/United States
- U10 CA180820/CA/NCI NIH HHS/United States
- U10 CA180888/CA/NCI NIH HHS/United States
- UG1 CA233290/CA/NCI NIH HHS/United States
- P30 CA093373/CA/NCI NIH HHS/United States
- UG1 CA233331/CA/NCI NIH HHS/United States
- U10 CA180863/CA/NCI NIH HHS/United States
- UG1 CA233180/CA/NCI NIH HHS/United States
- P30 CA023100/CA/NCI NIH HHS/United States
- UG1 CA233191/CA/NCI NIH HHS/United States

