Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care
- PMID: 32706859
- PMCID: PMC7454434
- DOI: 10.1093/cid/ciaa1041
Remdesivir for Severe Coronavirus Disease 2019 (COVID-19) Versus a Cohort Receiving Standard of Care
Abstract
Background: We compared the efficacy of the antiviral agent, remdesivir, versus standard-of-care treatment in adults with severe coronavirus disease 2019 (COVID-19) using data from a phase 3 remdesivir trial and a retrospective cohort of patients with severe COVID-19 treated with standard of care.
Methods: GS-US-540-5773 is an ongoing phase 3, randomized, open-label trial comparing two courses of remdesivir (remdesivir-cohort). GS-US-540-5807 is an ongoing real-world, retrospective cohort study of clinical outcomes in patients receiving standard-of-care treatment (non-remdesivir-cohort). Inclusion criteria were similar between studies: patients had confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, were hospitalized, had oxygen saturation ≤94% on room air or required supplemental oxygen, and had pulmonary infiltrates. Stabilized inverse probability of treatment weighted multivariable logistic regression was used to estimate the treatment effect of remdesivir versus standard of care. The primary endpoint was the proportion of patients with recovery on day 14, dichotomized from a 7-point clinical status ordinal scale. A key secondary endpoint was mortality.
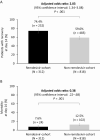
Results: After the inverse probability of treatment weighting procedure, 312 and 818 patients were counted in the remdesivir- and non-remdesivir-cohorts, respectively. At day 14, 74.4% of patients in the remdesivir-cohort had recovered versus 59.0% in the non-remdesivir-cohort (adjusted odds ratio [aOR] 2.03: 95% confidence interval [CI]: 1.34-3.08, P < .001). At day 14, 7.6% of patients in the remdesivir-cohort had died versus 12.5% in the non-remdesivir-cohort (aOR 0.38, 95% CI: .22-.68, P = .001).
Conclusions: In this comparative analysis, by day 14, remdesivir was associated with significantly greater recovery and 62% reduced odds of death versus standard-of-care treatment in patients with severe COVID-19.
Clinical trials registration: NCT04292899 and EUPAS34303.
Keywords: SARS-CoV-2; antiviral treatment; remdesivir; severe COVID-19.
© The Author(s) 2020. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
The Multidimensional Challenge of Treating Coronavirus Disease 2019 (COVID-19): Remdesivir Is a Foot in the Door.Clin Infect Dis. 2021 Dec 6;73(11):e4175-e4178. doi: 10.1093/cid/ciaa1132. Clin Infect Dis. 2021. PMID: 32735655 Free PMC article. No abstract available.
-
Call for Action: Racial Disparities in Clinical Research.Clin Infect Dis. 2021 Jul 15;73(2):356-357. doi: 10.1093/cid/ciaa1349. Clin Infect Dis. 2021. PMID: 32894751 No abstract available.
-
Reply to Pandita et al.Clin Infect Dis. 2021 Jul 15;73(2):357. doi: 10.1093/cid/ciaa1350. Clin Infect Dis. 2021. PMID: 32894754 Free PMC article. No abstract available.
References
-
- Johns Hopkins University and Medicine. Coronavirus resource center. Available at: https://coronavirus.jhu.edu/. Accessed 06 August 2020.
-
- US Food & Drug Administration. Remdesivir emergency use authorization letter. Available at: https://www.fda.gov/media/137564/download. Accessed 6 May 2020.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

