Mutations in MYLPF Cause a Novel Segmental Amyoplasia that Manifests as Distal Arthrogryposis
- PMID: 32707087
- PMCID: PMC7413889
- DOI: 10.1016/j.ajhg.2020.06.014
Mutations in MYLPF Cause a Novel Segmental Amyoplasia that Manifests as Distal Arthrogryposis
Abstract
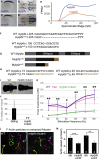
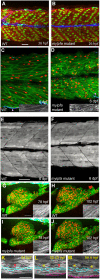
We identified ten persons in six consanguineous families with distal arthrogryposis (DA) who had congenital contractures, scoliosis, and short stature. Exome sequencing revealed that each affected person was homozygous for one of two different rare variants (c.470G>T [p.Cys157Phe] or c.469T>C [p.Cys157Arg]) affecting the same residue of myosin light chain, phosphorylatable, fast skeletal muscle (MYLPF). In a seventh family, a c.487G>A (p.Gly163Ser) variant in MYLPF arose de novo in a father, who transmitted it to his son. In an eighth family comprised of seven individuals with dominantly inherited DA, a c.98C>T (p.Ala33Val) variant segregated in all four persons tested. Variants in MYLPF underlie both dominant and recessively inherited DA. Mylpf protein models suggest that the residues associated with dominant DA interact with myosin whereas the residues altered in families with recessive DA only indirectly impair this interaction. Pathological and histological exam of a foot amputated from an affected child revealed complete absence of skeletal muscle (i.e., segmental amyoplasia). To investigate the mechanism for this finding, we generated an animal model for partial MYLPF impairment by knocking out zebrafish mylpfa. The mylpfa mutant had reduced trunk contractile force and complete pectoral fin paralysis, demonstrating that mylpf impairment most severely affects limb movement. mylpfa mutant muscle weakness was most pronounced in an appendicular muscle and was explained by reduced myosin activity and fiber degeneration. Collectively, our findings demonstrate that partial loss of MYLPF function can lead to congenital contractures, likely as a result of degeneration of skeletal muscle in the distal limb.
Keywords: Mendelian disease; amyoplasia; congenital contractures; development; distal arthrogryposis; exome sequencing; myosin; skeletal muscle; zebrafish.
Copyright © 2020 American Society of Human Genetics. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors declare no competing interests.
Figures



Comment in
-
Using the Term Amyoplasia Loosely Can Lead to Confusion.Am J Hum Genet. 2020 Dec 3;107(6):1186-1187. doi: 10.1016/j.ajhg.2020.10.014. Am J Hum Genet. 2020. PMID: 33275911 Free PMC article. No abstract available.
-
Response to Hall et al.Am J Hum Genet. 2020 Dec 3;107(6):1188-1189. doi: 10.1016/j.ajhg.2020.11.006. Am J Hum Genet. 2020. PMID: 33275912 Free PMC article. No abstract available.
References
-
- Bamshad M., Jorde L.B., Carey J.C. A revised and extended classification of the distal arthrogryposes. Am. J. Med. Genet. 1996;65:277–281. - PubMed
-
- Bamshad M., Bohnsack J.F., Jorde L.B., Carey J.C. Distal arthrogryposis type 1: clinical analysis of a large kindred. Am. J. Med. Genet. 1996;65:282–285. - PubMed
-
- Stevenson D.A., Carey J.C., Palumbos J., Rutherford A., Dolcourt J., Bamshad M.J. Clinical characteristics and natural history of Freeman-Sheldon syndrome. Pediatrics. 2006;117:754–762. - PubMed
-
- Krakowiak P.A., Bohnsack J.F., Carey J.C., Bamshad M. Clinical analysis of a variant of Freeman-Sheldon syndrome (DA2B) Am. J. Med. Genet. 1998;76:93–98. - PubMed
-
- Beck A.E., McMillin M.J., Gildersleeve H.I.S., Shively K.M.B., Tang A., Bamshad M.J. Genotype-phenotype relationships in Freeman-Sheldon syndrome. Am. J. Med. Genet. A. 2014;164A:2808–2813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL150953/HL/NHLBI NIH HHS/United States
- P30 NS045758/NS/NINDS NIH HHS/United States
- R01 GM088041/GM/NIGMS NIH HHS/United States
- P30 NS104177/NS/NINDS NIH HHS/United States
- S10 OD021553/OD/NIH HHS/United States
- U24 HG008956/HG/NHGRI NIH HHS/United States
- T32 NS077984/NS/NINDS NIH HHS/United States
- S10 OD010383/OD/NIH HHS/United States
- R01 HD048895/HD/NICHD NIH HHS/United States
- R01 GM117964/GM/NIGMS NIH HHS/United States
- UM1 HG006493/HG/NHGRI NIH HHS/United States
- R01 AR067279/AR/NIAMS NIH HHS/United States

