Micro and nanoscale technologies in oral drug delivery
- PMID: 32707147
- PMCID: PMC7374157
- DOI: 10.1016/j.addr.2020.07.012
Micro and nanoscale technologies in oral drug delivery
Abstract
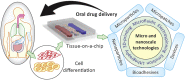
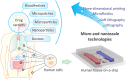
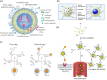
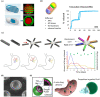
Oral administration is a pillar of the pharmaceutical industry and yet it remains challenging to administer hydrophilic therapeutics by the oral route. Smart and controlled oral drug delivery could bypass the physiological barriers that limit the oral delivery of these therapeutics. Micro- and nanoscale technologies, with an unprecedented ability to create, control, and measure micro- or nanoenvironments, have found tremendous applications in biology and medicine. In particular, significant advances have been made in using these technologies for oral drug delivery. In this review, we briefly describe biological barriers to oral drug delivery and micro and nanoscale fabrication technologies. Micro and nanoscale drug carriers fabricated using these technologies, including bioadhesives, microparticles, micropatches, and nanoparticles, are described. Other applications of micro and nanoscale technologies are discussed, including fabrication of devices and tissue engineering models to precisely control or assess oral drug delivery in vivo and in vitro, respectively. Strategies to advance translation of micro and nanotechnologies into clinical trials for oral drug delivery are mentioned. Finally, challenges and future prospects on further integration of micro and nanoscale technologies with oral drug delivery systems are highlighted.
Keywords: Drug delivery devices; Micro and nanocarriers; Micro and nanoscale technologies; Oral drug delivery; Tissue models.
Copyright © 2020. Published by Elsevier B.V.
Figures




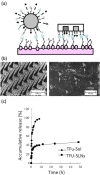

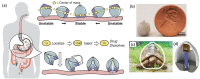
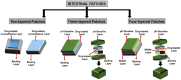




References
-
- Sastry S.V., Nyshadham J.R., Fix J.A. Recent technological advances in oral drug delivery–a review. Pharmaceut Sci Tech Today. 2000;3(4):138–145. - PubMed
-
- Maham A., Tang Z., Wu H., Wang J., Lin Y. Protein-based nanomedicine platforms for drug delivery. Small. 2009;5(15):1706–1721. - PubMed
-
- Koziolek M., Grimm M., Schneider F., Jedamzik P., Sager M., Kuhn J.P., Siegmund W., Weitschies W. Navigating the human gastrointestinal tract for oral drug delivery: uncharted waters and new frontiers. Adv. Drug Deliv. Rev. 2016;101:75–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

