CRISPR/Cas systems to overcome challenges in developing the next generation of T cells for cancer therapy
- PMID: 32707148
- PMCID: PMC7736063
- DOI: 10.1016/j.addr.2020.07.015
CRISPR/Cas systems to overcome challenges in developing the next generation of T cells for cancer therapy
Abstract
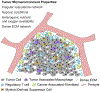
Genetically engineered immune cells with chimeric antigen receptors (CAR) or modified T cell receptors (TCR) have demonstrated their potential as a potent class of new cancer therapeutic strategy. Despite the clinical success of autologous CD19 CAR T cells in hematological malignancies, allogeneic T cells exhibit many advantages over their autologous counterparts and have recently gathered widespread attention due to the emergence of multiplex genome editing techniques, particularly CRISPR/Cas systems. Furthermore, genetically engineered T cells face a host of major challenges in solid tumors that are not as significant for blood cancers such as T cell targeted delivery, target specificity, proliferation, persistence, and the immunosuppressive tumor microenvironment. We take this opportunity to analyze recent strategies to develop allogeneic T cells, specifically in consideration of CRISPR/Cas and its delivery systems for multiplex gene editing. Additionally, we discuss the current methods used to delivery CRISPR/Cas systems for immunotherapeutic applications, and the challenges to continued development of novel delivery systems. We also provide a comprehensive analysis of the major challenges that genetically engineered T cells face in solid tumors along with the most recent strategies to overcome these barriers, with an emphasis on CRISPR-based approaches. We illustrate the synergistic prospects for how the combination of synthetic biology and immune-oncology could pave the way for designing the next generation of precision cancer therapy.
Keywords: Adoptive cell therapy; Allogeneic T cells; CRISPR/Cas delivery systems; Cancer immunotherapy; Chimeric antigen receptor; Solid tumor.
Copyright © 2020 Elsevier B.V. All rights reserved.
Figures
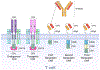


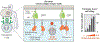

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

