SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons
- PMID: 32707511
- PMCID: PMC7336915
- DOI: 10.1016/j.jcv.2020.104542
SARS-CoV-2-IgG response is different in COVID-19 outpatients and asymptomatic contact persons
Abstract
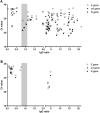
Commercially available immunoassays have been developed for sensitive and specific detection of antibodies against SARS-CoV-2. While a fast and reliable IgG response has been reported for samples from hospitalized COVID-19 patients, less is known about ambulatory patients. We evaluated the SARS-CoV-2-IgG response by the Anti-SARS-CoV-2-ELISA IgG (Euroimmun) in a defined cohort of SARS-CoV-2-PCR-confirmed outpatients and asymptomatic contact persons including 137 serum samples from PCR-confirmed outpatients (n = 111) and asymptomatic but PCR-positive contact persons (n = 26) sent to our laboratory as part of routine diagnostics for determination of SARS-CoV-2-IgG. Overall positivity rate for SARS-CoV-2-IgG was 81.1 % in outpatients (irrespective of sampling before or after day 21 after onset of symptoms) but significantly lower in asymptomatic contact persons (15.4 %, p < 0.0001). In contact persons without symptoms the ct values of the PCR assays were significantly higher (5-7 threshold cycles) than in outpatients, and ct values were significantly negative correlated to the SARS-CoV-2-IgG ratio, suggesting a lower viral load as a possible explanation for lower rate of seropositivity. In summary, our study shows that serological response to SARS-CoV-2 in outpatients including asymptomatic persons is less pronounced than in hospitalized patients. Further controlled studies are urgently needed to determine serological response in outpatients and asymptomatic persons since this is the main target population for seroepidemiological investigations.
Keywords: COVID-19; Contact persons; Outpatients; SARS-CoV-2-IgG; ct values.
Copyright © 2020 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
There are no conflicts of interest by all authors.
Figures
References
-
- Lassaunière R., Frische A., Harboe Z.B., Nielsen A.C.Y., Fomsgaard A., Krogfelt K.A. Evaluation of nine commercial SARS-CoV-2 immunoassays. medrxiv. 2020 doi:https://doiorg/101101/2020 04 0920056325.
-
- Tre-Hardy M., Wilmet A., Beukinga I., Dogne J.M., Douxfils J., Blairon L. Validation of a chemiluminescent assay for specific SARS-CoV-2 antibody. Clin. Chem. Lab. Med. 2020;(May 25) - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

