West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and "One Health" Implications
- PMID: 32707644
- PMCID: PMC7400489
- DOI: 10.3390/pathogens9070589
West Nile Virus: An Update on Pathobiology, Epidemiology, Diagnostics, Control and "One Health" Implications
Abstract
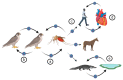
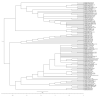
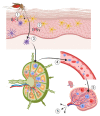
West Nile virus (WNV) is an important zoonotic flavivirus responsible for mild fever to severe, lethal neuroinvasive disease in humans, horses, birds, and other wildlife species. Since its discovery, WNV has caused multiple human and animal disease outbreaks in all continents, except Antarctica. Infections are associated with economic losses, mainly due to the cost of treatment of infected patients, control programmes, and loss of animals and animal products. The pathogenesis of WNV has been extensively investigated in natural hosts as well as in several animal models, including rodents, lagomorphs, birds, and reptiles. However, most of the proposed pathogenesis hypotheses remain contentious, and much remains to be elucidated. At the same time, the unavailability of specific antiviral treatment or effective and safe vaccines contribute to the perpetuation of the disease and regular occurrence of outbreaks in both endemic and non-endemic areas. Moreover, globalisation and climate change are also important drivers of the emergence and re-emergence of the virus and disease. Here, we give an update of the pathobiology, epidemiology, diagnostics, control, and "One Health" implications of WNV infection and disease.
Keywords: West Nile virus; control; one health; pathogenesis.
Conflict of interest statement
All authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish this review article.
Figures


References
-
- Phalen D.N., Dahlhausen B. West Nile virus. Semin. Avian Exot. Pet. Med. 2004;13:67–78. doi: 10.1053/j.saep.2004.01.002. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

