Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment
- PMID: 32707672
- PMCID: PMC7409093
- DOI: 10.3390/cancers12071960
Therapeutic Strategies for Overcoming Immunotherapy Resistance Mediated by Immunosuppressive Factors of the Glioblastoma Microenvironment
Abstract
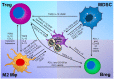
Various mechanisms of treatment resistance have been reported for glioblastoma (GBM) and other tumors. Resistance to immunotherapy in GBM patients may be caused by acquisition of immunosuppressive ability by tumor cells and an altered tumor microenvironment. Although novel strategies using an immune-checkpoint inhibitor (ICI), such as anti-programmed cell death-1 antibody, have been clinically proven to be effective in many types of malignant tumors, such strategies may be insufficient to prevent regrowth in recurrent GBM. The main cause of GBM recurrence may be the existence of an immunosuppressive tumor microenvironment involving immunosuppressive cytokines, extracellular vesicles, chemokines produced by glioma and glioma-initiating cells, immunosuppressive cells, etc. Among these, recent research has paid attention to various immunosuppressive cells-including M2-type macrophages and myeloid-derived suppressor cells-that cause immunosuppression in GBM microenvironments. Here, we review the epidemiological features, tumor immune microenvironment, and associations between the expression of immune checkpoint molecules and the prognosis of GBM. We also reviewed various ongoing or future immunotherapies for GBM. Various strategies, such as a combination of ICI therapies, might overcome these immunosuppressive mechanisms in the GBM microenvironment.
Keywords: M2-type macrophages; glioma; immune-checkpoint molecules; immunosuppressive tumor microenvironment; tumor vaccine.
Conflict of interest statement
The authors have no conflicts of interest directly relevant to the content of this article. As indirect relevance, some of the materials for the AFTV described in this review article was provided by Cell-Medicine, Inc. (CMI), which is a venture company for research and development of immunotherapy born from RIKEN (The Institute of Physical and Chemical Research) and University of Tsukuba in Japan. T.M. is a member of CMI.
Figures



References
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. - DOI - PubMed
-
- Gorlia T., Stupp R., Brandes A.A., Rampling R.R., Fumoleau P., Dittrich C., Campone M.M., Twelves C.C., Raymond E., Hegi M.E., et al. New prognostic factors and calculators for outcome prediction in patients with recurrent glioblastoma: A pooled analysis of EORTC Brain Tumour Group phase I and II clinical trials. Eur. J. Cancer. 2012;48:1176–1184. doi: 10.1016/j.ejca.2012.02.004. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

