The Emerging Role of Extracellular Vesicles in the Glioma Microenvironment: Biogenesis and Clinical Relevance
- PMID: 32707733
- PMCID: PMC7409063
- DOI: 10.3390/cancers12071964
The Emerging Role of Extracellular Vesicles in the Glioma Microenvironment: Biogenesis and Clinical Relevance
Abstract
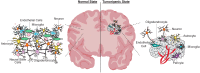
Gliomas are a diverse group of brain tumors comprised of malignant cells ('tumor' cells) and non-malignant 'normal' cells, including neural (neurons, glia), inflammatory (microglia, macrophage) and vascular cells. Tumor heterogeneity arises in part because, within the glioma mass, both 'tumor' and 'normal' cells secrete factors that form a unique microenvironment to influence tumor progression. Extracellular vesicles (EVs) are critical mediators of intercellular communication between immediate cellular neighbors and distantly located cells in healthy tissues/organs and in tumors, including gliomas. EVs mediate cell-cell signaling as carriers of nucleic acid, lipid and protein cargo, and their content is unique to cell types and physiological states. EVs secreted by non-malignant neural cells have important physiological roles in the healthy brain, which can be altered or co-opted to promote tumor progression and metastasis, acting in combination with glioma-secreted EVs. The cell-type specificity of EV content means that 'vesiculome' data can potentially be used to trace the cell of origin. EVs may also serve as biomarkers to be exploited for disease diagnosis and to assess therapeutic progress. In this review, we discuss how EVs mediate intercellular communication in glioma, and their potential role as biomarkers and readouts of a therapeutic response.
Keywords: biomarkers; extracellular vesicles; glioma; liquid biopsy; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Johnstone R.M., Adam M., Hammond J.R., Orr L., Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) J. Biol. Chem. 1987;262:9412–9420. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

