Characterization of Fluid Biomarkers Reveals Lysosome Dysfunction and Neurodegeneration in Neuronopathic MPS II Patients
- PMID: 32707880
- PMCID: PMC7432645
- DOI: 10.3390/ijms21155188
Characterization of Fluid Biomarkers Reveals Lysosome Dysfunction and Neurodegeneration in Neuronopathic MPS II Patients
Abstract
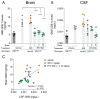
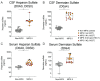
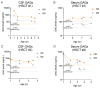
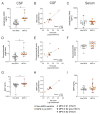
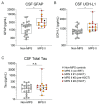
Mucopolysaccharidosis type II is a lysosomal storage disorder caused by a deficiency of iduronate-2-sulfatase (IDS) and characterized by the accumulation of the primary storage substrate, glycosaminoglycans (GAGs). Understanding central nervous system (CNS) pathophysiology in neuronopathic MPS II (nMPS II) has been hindered by the lack of CNS biomarkers. Characterization of fluid biomarkers has been largely focused on evaluating GAGs in cerebrospinal fluid (CSF) and the periphery; however, GAG levels alone do not accurately reflect the broad cellular dysfunction in the brains of MPS II patients. We utilized a preclinical mouse model of MPS II, treated with a brain penetrant form of IDS (ETV:IDS) to establish the relationship between markers of primary storage and downstream pathway biomarkers in the brain and CSF. We extended the characterization of pathway and neurodegeneration biomarkers to nMPS II patient samples. In addition to the accumulation of CSF GAGs, nMPS II patients show elevated levels of lysosomal lipids, neurofilament light chain, and other biomarkers of neuronal damage and degeneration. Furthermore, we find that these biomarkers of downstream pathology are tightly correlated with heparan sulfate. Exploration of the responsiveness of not only CSF GAGs but also pathway and disease-relevant biomarkers during drug development will be crucial for monitoring disease progression, and the development of effective therapies for nMPS II.
Keywords: ETV:IDS; GM3; Hunter syndrome; biomarkers; cerebrospinal fluid; dermatan sulfate (DS); gangliosides; glycosaminoglycans (GAGs); heparan sulfate (HS); inflammation; lysosome dysfunction; mucopolysaccharidosis type II; neurodegeneration; neurofilament light chain (Nf-L).
Conflict of interest statement
M.E. is a consultant for Takeda, REGENXBIO, and Denali Therapeutics.
Figures




References
-
- Lampe C., Atherton A., Burton B.K., Descartes M., Giugliani R., Horovitz D.D., Kyosen S.O., Magalhães T.S., Martins A.M., Mendelsohn N.J., et al. Enzyme Replacement Therapy in Mucopolysaccharidosis II Patients Under 1 Year of Age. JIMD Rep. 2014;14:99–113. doi: 10.1007/8904_2013_289. - DOI - PMC - PubMed
-
- Hendriksz C.J., Muenzer J., Vanderver A., Davis J.M., Burton B.K., Mendelsohn N.J., Wang N., Pan L., Pano A., Barbier A.J. Levels of glycosaminoglycans in the cerebrospinal fluid of healthy young adults, surrogate-normal children, and Hunter syndrome patients with and without cognitive impairment. Mol. Genet. Metab. Rep. 2015;5:103–106. doi: 10.1016/j.ymgmr.2015.11.001. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

