Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Sensitive, Specific, and Cost-Effective Point-of-Care Test for Coronaviruses in the Context of COVID-19 Pandemic
- PMID: 32707972
- PMCID: PMC7464797
- DOI: 10.3390/biology9080182
Loop-Mediated Isothermal Amplification (LAMP): A Rapid, Sensitive, Specific, and Cost-Effective Point-of-Care Test for Coronaviruses in the Context of COVID-19 Pandemic
Abstract
The rampant spread of COVID-19 and the worldwide prevalence of infected cases demand a rapid, simple, and cost-effective Point of Care Test (PoCT) for the accurate diagnosis of this pandemic. The most common molecular tests approved by regulatory bodies across the world for COVID-19 diagnosis are based on Polymerase Chain Reaction (PCR). While PCR-based tests are highly sensitive, specific, and remarkably reliable, they have many limitations ranging from the requirement of sophisticated laboratories, need of skilled personnel, use of complex protocol, long wait times for results, and an overall high cost per test. These limitations have inspired researchers to search for alternative diagnostic methods that are fast, economical, and executable in low-resource laboratory settings. The discovery of Loop-mediated isothermal Amplification (LAMP) has provided a reliable substitute platform for the accurate detection of low copy number nucleic acids in the diagnosis of several viral diseases, including epidemics like Severe Acute Respiratory Syndrome (SARS) and Middle East Respiratory Syndrome (MERS). At present, a cocktail of LAMP assay reagents along with reverse transcriptase enzyme (Reverse Transcription LAMP, RT-LAMP) can be a robust solution for the rapid and cost-effective diagnosis for COVID-19, particularly in developing, and low-income countries. In summary, the development of RT-LAMP based diagnostic tools in a paper/strip format or the integration of this method into a microfluidic platform such as a Lab-on-a-chip may revolutionize the concept of PoCT for COVID-19 diagnosis. This review discusses the principle, technology and past research underpinning the success for using this method for diagnosing MERS and SARS, in addition to ongoing research, and the prominent prospect of RT-LAMP in the context of COVID-19 diagnosis.
Keywords: COVID-19; LAMP; RT-LAMP; SARS-CoV-2; coronavirus; point-of-care tests; reverse transcription loop-mediated isothermal amplification.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

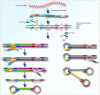


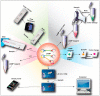
References
-
- Nejadi Babadaei M.M., Hasan A., Haj Bloukh S., Edis Z., Sharifi M., Kachooei E., Falahati M. The expression level of angiotensin-converting enzyme 2 determine the severity of COVID-19: Lung and heart tissue as targets. J. Biomol. Struct. Dyn. 2020 doi: 10.1080/07391102.2020.1767211. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

