Oropouche Virus Infects, Persists and Induces IFN Response in Human Peripheral Blood Mononuclear Cells as Identified by RNA PrimeFlow™ and qRT-PCR Assays
- PMID: 32708342
- PMCID: PMC7411765
- DOI: 10.3390/v12070785
Oropouche Virus Infects, Persists and Induces IFN Response in Human Peripheral Blood Mononuclear Cells as Identified by RNA PrimeFlow™ and qRT-PCR Assays
Abstract
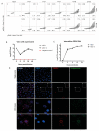
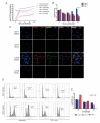
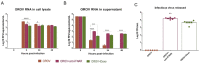
Oropouche orthobunyavirus (OROV) is an emerging arbovirus with a high potential of dissemination in America. Little is known about the role of peripheral blood mononuclear cells (PBMC) response during OROV infection in humans. Thus, to evaluate human leukocytes susceptibility, permissiveness and immune response during OROV infection, we applied RNA hybridization, qRT-PCR and cell-based assays to quantify viral antigens, genome, antigenome and gene expression in different cells. First, we observed OROV replication in human leukocytes lineages as THP-1 monocytes, Jeko-1 B cells and Jurkat T cells. Interestingly, cell viability and viral particle detection are maintained in these cells, even after successive passages. PBMCs from healthy donors were susceptible but the infection was not productive, since neither antigenome nor infectious particle was found in the supernatant of infected PBMCs. In fact, only viral antigens and small quantities of OROV genome were detected at 24 hpi in lymphocytes, monocytes and CD11c+ cells. Finally, activation of the Interferon (IFN) response was essential to restrict OROV replication in human PBMCs. Increased expression of type I/III IFNs, ISGs and inflammatory cytokines was detected in the first 24 hpi and viral replication was re-established after blocking IFNAR or treating cells with glucocorticoid. Thus, in short, our results show OROV is able to infect and remain in low titers in human T cells, monocytes, DCs and B cells as a consequence of an effective IFN response after infection, indicating the possibility of leukocytes serving as a trojan horse in specific microenvironments during immunosuppression.
Keywords: B cells; Oropouche virus; PBMC; RNA PrimeFlow™; dendritic cells; interferons; lymphocyte; monocytes.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Travassos Da Rosa J.F., De Souza W.M., De Paula Pinheiro F., Figueiredo M.L., Cardoso J.F., Acrani G.O., Teixeira Nunes M.R. Oropouche virus: Clinical, epidemiological, and molecular aspects of a neglected orthobunyavirus. Am. J. Trop. Med. Hyg. 2017;96:1019–1030. doi: 10.4269/ajtmh.16-0672. - DOI - PMC - PubMed
-
- de Souza Luna L.K., Rodrigues A.H., Santos R.I.M., Sesti-Costa R., Criado M.F., Martins R.B., Silva M.L., Delcaro L.S., Proença-Modena J.L., Figueiredo L.T.M., et al. Oropouche Virus Is Detected in Peripheral Blood Leukocytes From Patients. J. Med. Virol. 2017;89:1108–1111. doi: 10.1002/jmv.24722. - DOI - PubMed
-
- Cardoso B.F., Serra O.P., Da Silva Heinen L.B., Zuchi N., De Souza V.C., Naveca F.G., Dos Santos M.A.M., Slhessarenko R.D. Detection of oropouche virus segment s in patients and in culex quinquefasciatus in the state of mato grosso, Brazil. Mem. Inst. Oswaldo Cruz. 2015;110:745–754. doi: 10.1590/0074-02760150123. - DOI - PMC - PubMed
-
- Vasconcelos H.B., Azevedo R.S.S., Casseb S.M., Nunes-neto J.P., Chiang J.O., Cantuária P.C., Segura M.N.O., Martins L.C., Monteiro H.A.O., Rodrigues S.G., et al. Oropouche fever epidemic in Northern Brazil: Epidemiology and molecular characterization of isolates. J. Clin. Virol. 2008;44:129–133. doi: 10.1016/j.jcv.2008.11.006. - DOI - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials

