Selenium Deficiency Is Associated with Mortality Risk from COVID-19
- PMID: 32708526
- PMCID: PMC7400921
- DOI: 10.3390/nu12072098
Selenium Deficiency Is Associated with Mortality Risk from COVID-19
Abstract
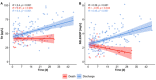
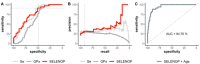
SARS-CoV-2 infections underlie the current coronavirus disease (COVID-19) pandemic and are causative for a high death toll particularly among elderly subjects and those with comorbidities. Selenium (Se) is an essential trace element of high importance for human health and particularly for a well-balanced immune response. The mortality risk from a severe disease like sepsis or polytrauma is inversely related to Se status. We hypothesized that this relation also applies to COVID-19. Serum samples (n = 166) from COVID-19 patients (n = 33) were collected consecutively and analyzed for total Se by X-ray fluorescence and selenoprotein P (SELENOP) by a validated ELISA. Both biomarkers showed the expected strong correlation (r = 0.7758, p < 0.001), pointing to an insufficient Se availability for optimal selenoprotein expression. In comparison with reference data from a European cross-sectional analysis (EPIC, n = 1915), the patients showed a pronounced deficit in total serum Se (mean ± SD, 50.8 ± 15.7 vs. 84.4 ± 23.4 µg/L) and SELENOP (3.0 ± 1.4 vs. 4.3 ± 1.0 mg/L) concentrations. A Se status below the 2.5th percentile of the reference population, i.e., [Se] < 45.7 µg/L and [SELENOP] < 2.56 mg/L, was present in 43.4% and 39.2% of COVID samples, respectively. The Se status was significantly higher in samples from surviving COVID patients as compared with non-survivors (Se; 53.3 ± 16.2 vs. 40.8 ± 8.1 µg/L, SELENOP; 3.3 ± 1.3 vs. 2.1 ± 0.9 mg/L), recovering with time in survivors while remaining low or even declining in non-survivors. We conclude that Se status analysis in COVID patients provides diagnostic information. However, causality remains unknown due to the observational nature of this study. Nevertheless, the findings strengthen the notion of a relevant role of Se for COVID convalescence and support the discussion on adjuvant Se supplementation in severely diseased and Se-deficient patients.
Keywords: COVID-19; inflammation; micronutrient; selenoprotein P; trace element.
Conflict of interest statement
L.S. holds shares and P.S. serves as CEO of selenOmed GmbH, a company involved in Se status assessment and supplementation. The other authors declare no competing interest.
Figures



References
-
- Villar J., Ferrando C., Martinez D., Ambros A., Munoz T., Soler J.A., Aguilar G., Alba F., Gonzalez-Higueras E., Conesa L.A., et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

