Continuous Flow Synthesis of Heterocycles: A Recent Update on the Flow Synthesis of Indoles
- PMID: 32708643
- PMCID: PMC7397031
- DOI: 10.3390/molecules25143242
Continuous Flow Synthesis of Heterocycles: A Recent Update on the Flow Synthesis of Indoles
Abstract
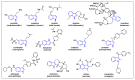
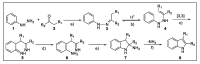
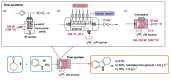
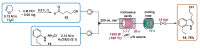
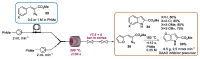
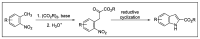
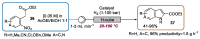
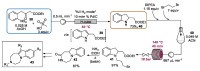
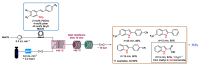
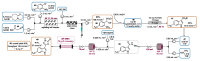
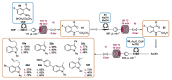
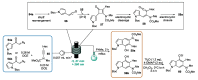
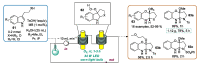
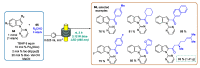
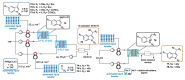
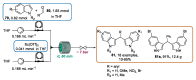
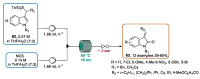
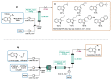
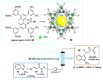
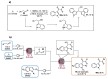
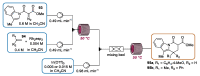
Indole derivatives are among the most useful and interesting heterocycles employed in drug discovery and medicinal chemistry. In addition, flow chemistry and flow technology are changing the synthetic paradigm in the field of modern synthesis. In this review, the role of flow technology in the preparation of indole derivatives is showcased. Selected examples have been described with the aim to provide readers with an overview on the tactics and technologies used for targeting indole scaffolds.
Keywords: flow chemistry; indoles; organic synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
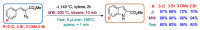


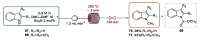
Similar articles
-
Indole as a Versatile Building Block in Cycloaddition Reactions: Synthesis of Diverse Heterocyclic Frameworks.Top Curr Chem (Cham). 2024 May 17;382(2):18. doi: 10.1007/s41061-024-00463-y. Top Curr Chem (Cham). 2024. PMID: 38758483 Review.
-
The flow synthesis of heterocycles for natural product and medicinal chemistry applications.Mol Divers. 2011 Aug;15(3):613-30. doi: 10.1007/s11030-010-9282-1. Epub 2010 Oct 20. Mol Divers. 2011. PMID: 20960230
-
Synthesis of indole-annulated sulfur heterocycles using copper-catalysed C-N coupling and palladium-catalysed direct arylation.Org Biomol Chem. 2016 Apr 7;14(13):3450-8. doi: 10.1039/c6ob00117c. Epub 2016 Mar 11. Org Biomol Chem. 2016. PMID: 26964635
-
Design, synthesis, and structure-activity relationships of indole-3-heterocycles as agonists of the CB1 receptor.Bioorg Med Chem Lett. 2011 Jan 1;21(1):506-9. doi: 10.1016/j.bmcl.2010.10.093. Epub 2010 Oct 25. Bioorg Med Chem Lett. 2011. PMID: 21075630
-
Recent advances in the synthesis of indole and quinoline derivatives through cascade reactions.Chem Asian J. 2009 Jul 6;4(7):1036-48. doi: 10.1002/asia.200900018. Chem Asian J. 2009. PMID: 19360759 Review.
Cited by
-
Continuous flow synthesis of N,N-dimethyltryptamine (DMT) analogues with therapeutic potential.RSC Med Chem. 2024 Oct 7;16(1):367-72. doi: 10.1039/d4md00562g. Online ahead of print. RSC Med Chem. 2024. PMID: 39502869 Free PMC article.
-
Design, Synthesis, Anticancer Activity, and Solid Lipid Nanoparticle Formulation of Indole- and Benzimidazole-Based Compounds as Pro-Apoptotic Agents Targeting Bcl-2 Protein.Pharmaceuticals (Basel). 2021 Feb 1;14(2):113. doi: 10.3390/ph14020113. Pharmaceuticals (Basel). 2021. PMID: 33535550 Free PMC article.
-
Development and Characterization of Indole-Responsive Whole-Cell Biosensor Based on the Inducible Gene Expression System from Pseudomonas putida KT2440.Int J Mol Sci. 2022 Apr 22;23(9):4649. doi: 10.3390/ijms23094649. Int J Mol Sci. 2022. PMID: 35563040 Free PMC article.
-
Synthesis of 2-Substitued Indoles via Pd-Catalysed Cyclization in an Aqueous Micellar Medium.Molecules. 2021 Jun 26;26(13):3917. doi: 10.3390/molecules26133917. Molecules. 2021. PMID: 34206877 Free PMC article.
References
-
- Gribble G.W. Indole Ring Synthesis: From Natural Products to Drug Discovery. Wiley-VCH; Chichester, UK: 2016.
-
- Ghinea I.O., Dinica R.D. Breakthoughts in Indole and Indolizine Chemistry, New Synthetic Pathways, New Applications. In: Varala V., editor. Scope of Selective Heterocycles from Organic and Pharmaceutical Perspective. Intech Open; Rijeka, Croatia: 2016. pp. 1043–1561. Chapter 5.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

