Lower Energy Intake among Advanced vs. Early Parkinson's Disease Patients and Healthy Controls in a Clinical Lunch Setting: A Cross-Sectional Study
- PMID: 32708668
- PMCID: PMC7400863
- DOI: 10.3390/nu12072109
Lower Energy Intake among Advanced vs. Early Parkinson's Disease Patients and Healthy Controls in a Clinical Lunch Setting: A Cross-Sectional Study
Abstract
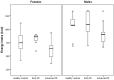
Unintentional weight loss has been observed among Parkinson's disease (PD) patients. Changes in energy intake (EI) and eating behavior, potentially caused by fine motor dysfunction and eating-related symptoms, might contribute to this. The primary aim of this study was to investigate differences in objectively measured EI between groups of healthy controls (HC), early (ESPD) and advanced stage PD patients (ASPD) during a standardized lunch in a clinical setting. The secondary aim was to identify clinical features and eating behavior abnormalities that explain EI differences. All participants (n = 23 HC, n = 20 ESPD, and n = 21 ASPD) went through clinical evaluations and were eating a standardized meal (200 g sausages, 400 g potato salad, 200 g apple purée and 500 mL water) in front of two video cameras. Participants ate freely, and the food was weighed pre- and post-meal to calculate EI (kcal). Multiple linear regression was used to explain group differences in EI. ASPD had a significantly lower EI vs. HC (-162 kcal, p < 0.05) and vs. ESPD (-203 kcal, p < 0.01) when controlling for sex. The number of spoonfuls, eating problems, dysphagia and upper extremity tremor could explain most (86%) of the lower EI vs. HC, while the first three could explain ~50% vs. ESPD. Food component intake analysis revealed significantly lower potato salad and sausage intakes among ASPD vs. both HC and ESPD, while water intake was lower vs. HC. EI is an important clinical target for PD patients with an increased risk of weight loss. Our results suggest that interventions targeting upper extremity tremor, spoonfuls, dysphagia and eating problems might be clinically useful in the prevention of unintentional weight loss in PD. Since EI was lower in ASPD, EI might be a useful marker of disease progression in PD.
Keywords: Parkinson’s disease; eating behavior; energy intake; food; malnutrition; monitoring; neurodegenerative diseases; weight loss.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Chaudhuri K.R., Martinez-Martin P., Schapira A.H.V., Stocchi F., Sethi K., Odin P., Brown R.G., Koller W., Barone P., MacPhee G., et al. International multicenter pilot study of the first comprehensive self-completed nonmotor symptoms questionnaire for Parkinson’s disease: The NMS Quest study. Mov. Disord. 2006;21:916–923. doi: 10.1002/mds.20844. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

