Sourdough Fermentation Degrades Wheat Alpha-Amylase/Trypsin Inhibitor (ATI) and Reduces Pro-Inflammatory Activity
- PMID: 32708800
- PMCID: PMC7404469
- DOI: 10.3390/foods9070943
Sourdough Fermentation Degrades Wheat Alpha-Amylase/Trypsin Inhibitor (ATI) and Reduces Pro-Inflammatory Activity
Abstract
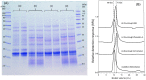
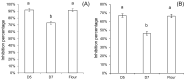
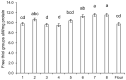
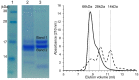
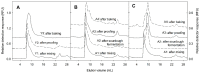
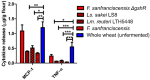
The ingestion of gluten-containing foods can cause wheat-related disorders in up to 15% of wheat consuming populations. Besides the role of gluten, α-amylase/trypsin inhibitors (ATI) have recently been identified as inducers of an innate immune response via toll-like receptor 4 in celiac disease and non-celiac wheat sensitivity. ATI are involved in plant self-defense against insects and possibly in grain development. Notably, they are largely resistant to gastrointestinal proteases and heat, and their inflammatory activity affects not only the intestine, but also peripheral organs. The aim of this study was to understand the changes of ATI throughout the sourdough and yeast-fermented bread-making processes. ATI tetramers were isolated, fluorescein-labelled, and added to a mini-dough bread-making system. When the pH decreased below 4.0 in sourdough fermentation, the ATI tetramers were degraded due to the activation of aspartic proteases, whilst in yeast fermentation, ATI tetramers remained intact. The amylase inhibitory activity after sourdough fermentation decreased significantly, while the concentration of free thiol groups increased. The glutathione reductase activity of Fructilactobacillus sanfranciscensis did not contribute to the reduction of ATI tetramers. Compared to the unfermented wheat, sourdough fermentation was able to decrease the release of pro-inflammatory cytokines monocyte chemoattractant protein-1 (MCP-1) and tumor necrosis factor alpha (TNF-α) in quantitative ATI extracts added to the human monocytic cell line THP-1. The current data suggest that sourdough fermentation can degrade ATI structure and bioactivity, and point to strategies to improve product development for wheat sensitivity patients.
Keywords: bioactivity; fermentation; innate immunity; lactic acid bacteria; wheat sensitivity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Catassi C., Alaedini A., Bojarski C., Bonaz B., Bouma G., Carroccio A., Castillejo G., De Magistris L., Dieterich W., Di Liberto D., et al. The Overlapping Area of Non-Celiac Gluten Sensitivity (NCGS) and Wheat-Sensitive Irritable Bowel Syndrome (IBS): An Update. Nutrients. 2017;9:1268. doi: 10.3390/nu9111268. - DOI - PMC - PubMed
-
- Fritscher-Ravens A., Schuppan D., Ellrichmann M., Schoch S., Röcken C., Brasch J., Bethge J., Böttner M., Klose J., Milla P.J. Confocal Endomicroscopy Shows Food-Associated Changes in the Intestinal Mucosa of Patients With Irritable Bowel Syndrome. Gastroenterology. 2014;147:1012–1020.e4. doi: 10.1053/j.gastro.2014.07.046. - DOI - PubMed
-
- Fritscher-Ravens A., Pflaum T., Mösinger M., Ruchay Z., Röcken C., Milla P.J., Das M., Böttner M., Wedel T., Schuppan D. Many Patients With Irritable Bowel Syndrome Have Atypical Food Allergies Not Associated With Immunoglobulin E. Gastroenterology. 2019;157:109–118.e5. doi: 10.1053/j.gastro.2019.03.046. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

