Exploring Metabolic Signature of Protein Energy Wasting in Hemodialysis Patients
- PMID: 32708829
- PMCID: PMC7408592
- DOI: 10.3390/metabo10070291
Exploring Metabolic Signature of Protein Energy Wasting in Hemodialysis Patients
Abstract
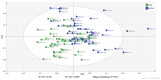
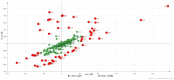
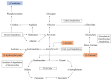
End-stage renal disease patients undergoing maintenance hemodialysis (HD) are vulnerable to the protein energy wasting (PEW) syndrome. Identification and diagnosis of PEW relies on clinical processes of judgment dependent on fulfilling multiple criteria drawn from serum biochemistry, weight status, predictive muscle mass, dietary energy and protein intakes. Therefore, we sought to explore the biomarkers' signature with plasma metabolites of PEW by using 1H-nuclear magnetic resonance for an untargeted metabolomics approach in the HD population, to understand metabolic alteration of PEW. In this case-controlled study, a total of 53 patients undergoing chronic HD were identified having PEW based on established diagnostic criteria and were age- and sex-matched with non-PEW (n = 53) HD patients. Fasting predialysis plasma samples were analyzed. Partial least square discriminant analysis demonstrated a significant separation between groups for specific metabolic pattern alterations. Further quantitative analysis showed that the level of 3-hydroxybutyrate, acetate, arabinose, maltose, ribose, sucrose and tartrate were significantly increased whilst creatinine was significantly decreased (all p < 0.05) in PEW subjects. Pathway analysis indicated that PEW-related metabolites reflected perturbations in fatty acid mechanism and induction of glyoxylate and dicarboxylate pathway attributed to gluconeogenesis. These results provide preliminary data in understanding metabolic alteration of PEW and corresponding abnormal metabolites that could potentially serve as biomarkers of PEW.
Keywords: 1H-NMR; hemodialysis; metabolic pathways; metabolomics; plasma metabolites; protein energy wasting.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Carrero J.J., Stenvinkel P., Cuppari L., Ikizler T.A., Kalantar-Zadeh K., Kaysen G., Mitch W.E., Price S.R., Wanner C., Wang A.Y.M., et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM) J. Ren. Nutr. 2013;23:77–90. doi: 10.1053/j.jrn.2013.01.001. - DOI - PubMed
-
- Gracia-Iguacel C., González-Parra E., Barril-Cuadrado G., Sánchez R., Egido J., Ortiz-Arduán A., Carrero J.J. Defining protein-energy wasting syndrome in chronic kidney disease: Prevalence and clinical implications. Nefrologia. 2014;34:507–519. doi: 10.3265/Nefrologia.pre2014.Apr.12522. - DOI - PubMed
-
- Lopes A.A., Bragg-Gresham J.L., Elder S.J., Ginsberg N., Goodkin D.A., Pifer T., Lameire N., Marshall M.R., Asano Y., Akizawa T. Independent and joint associations of nutritional status indicators with mortality risk among chronic hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study (DOPPS) J. Ren. Nutr. 2010;20:224–234. doi: 10.1053/j.jrn.2009.10.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources

