Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption
- PMID: 32708955
- PMCID: PMC7412034
- DOI: 10.3390/ma13143195
Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption
Abstract
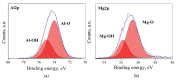
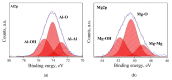
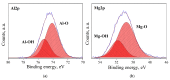
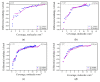
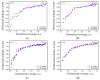
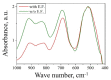
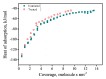
MgAl2O4 is used in humidity sensing and measurement, and as a catalyst or catalyst support in a wide variety of applications. For such applications, a detailed understanding of the surface properties and defect structure of the spinel, and, in particular, of the gas interactions at the spinel surface is essential. However, to the best of our knowledge, very limited experimental data regarding this subject is currently available. In this work, four spinel samples with an Al2O3 to MgO ratio (n) between 0.95 and 2.45 were synthesized and analyzed using X-ray photoelectron spectroscopy and water adsorption micro-calorimetry. The results showed that the spinel composition and its consequent defect structure do indeed have a distinct effect on the spinel-water vapor surface interactions. The adsorption behavior at the spinel-water interface showed changes that resulted from alterations in types and energetic diversity of adsorption sites, affecting both H2O uptake and overall energetics. Furthermore, changes in composition following appropriate thermal treatment were shown to have a major effect on the reducibility of the spinel which enabled increased water uptake at the surface. In addition to non-stoichiometry, the impact of intrinsic anti-site defects on the water-surface interaction was investigated. These defects were also shown to promote water uptake. Our results show that by composition modification and subsequent thermal treatments, the defect structure can be modified and controlled, allowing for the possibility of specifically designed spinels for water interactions.
Keywords: defect structure; magnesium aluminate spinel; reducibility; water adsorption.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Gusmano G., Montesperelli G., Traversa E., Mattogno G. Microstructure and electrical properties of MgAl2O4 thin films for humidity sensing. J. Am. Ceram. Soc. 1993;76:743–750. doi: 10.1111/j.1151-2916.1993.tb03669.x. - DOI
-
- Govindaraj A., Flahaut E., Laurent C., Peigney A., Rousset A., Rao C.N.R. An investigation of carbon nanotubes obtained from the decomposition of methane over reduced Mg1−xMxAl2O4 spinel catalysts. J. Mater. Res. 1999;14:2567–2576. doi: 10.1557/JMR.1999.0344. - DOI
-
- Mei D., Lebarbier Dagle V., Xing R., Albrecht K.O., Dagle R.A. Steam reforming of ethylene glycol over MgAl2O4 supported Rh, Ni, and Co Catalysts. ACS Catal. 2016;6:315–325. doi: 10.1021/acscatal.5b01666. - DOI
-
- Villa A., Gaiassi A., Rossetti I., Bianchi C.L., Van Benthem K., Veith G.M., Prati L. Au on MgAl2O4 spinels: The effect of support surface properties in glycerol oxidation. J. Catal. 2010;275:108–116. doi: 10.1016/j.jcat.2010.07.022. - DOI
-
- Mei D., Glezakou V.A., Lebarbier V., Kovarik L., Wan H., Albrecht K.O., Gerber M., Rousseau R., Dagle R.A. Highly active and stable MgAl2O4-supported Rh and Ir catalysts for methane steam reforming: A combined experimental and theoretical study. J. Catal. 2014;316:11–23. doi: 10.1016/j.jcat.2014.04.021. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

