Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures
- PMID: 32708967
- PMCID: PMC7407990
- DOI: 10.3390/nano10071392
Current Trends in Nanomaterials for Metal Oxide-Based Conductometric Gas Sensors: Advantages and Limitations. Part 1: 1D and 2D Nanostructures
Abstract
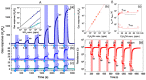
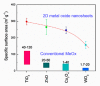
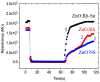
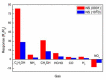
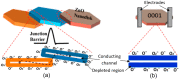
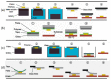
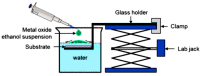
This article discusses the main uses of 1D and 2D nanomaterials in the development of conductometric gas sensors based on metal oxides. It is shown that, along with the advantages of these materials, which can improve the parameters of gas sensors, there are a number of disadvantages that significantly limit their use in the development of devices designed for the sensor market.
Keywords: fabrication; improvement; individual 1D structures; nanobelts; nanosheets; nanowire array; nanowires; stability; synthesis.
Conflict of interest statement
The author declare no conflict of interest.
Figures






































References
-
- Lu J.G., Chang P.C., Fan Z.Y. Quasi-one-dimensional metal oxide materials—Synthesis, properties and applications. Mater. Sci. Eng. R. 2006;52:49–91. doi: 10.1016/j.mser.2006.04.002. - DOI
-
- Comini E., Baratto C., Faglia G., Ferroni M., Vomiero A., Sberveglieri G. Quasi-one dimensional metal oxide semiconductors: Preparation, characterization and application as chemical sensors. Prog. Mater. Sci. 2009;54:1–67. doi: 10.1016/j.pmatsci.2008.06.003. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

