"To Anticipate": Neoadjuvant Therapy in Melanoma with a Focus on Predictive Biomarkers
- PMID: 32708968
- PMCID: PMC7409214
- DOI: 10.3390/cancers12071941
"To Anticipate": Neoadjuvant Therapy in Melanoma with a Focus on Predictive Biomarkers
Abstract
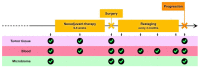
Despite surgical resection and adjuvant therapies, stage III melanomas still have a substantial risk of relapse. Neoadjuvant therapy is an emerging strategy that might offer superior efficacy compared to adjuvant therapy. Moreover, neoadjuvant therapy has some virtual advantages: it might allow for less demolitive surgery, permit the in vivo evaluation of drug efficacy, help tailor adjuvant treatments, and play a crucial role in innovative translational research. Herein, we review the available literature to explore the scientific background behind the neoadjuvant approach. We also discuss published clinical trials with a focus on predictive biomarkers and ongoing studies. Finally, we outline a possible framework for future neoadjuvant clinical trial development based on the International Neoadjuvant Melanoma Consortium guidelines.
Keywords: BRAF; MEK; adjuvant; immunotherapy; melanoma; neoadjuvant.
Conflict of interest statement
The authors declare no conflict of interest regarding this work. Alessandro Marco Minisini, reports an advisory role for Novartis, MSD, Pierre Fabre. Fabio Puglisi, reports grants from AstraZeneca, grants, personal fees and others from Roche, personal fees and others from Eli Lilly, personal fees from Amgen, personal fees from Ipsen, personal fees from MSD, personal fees from Takeda, grants and others from Eisai, others from Novartis, and others from Pfizer, outside the submitted work.
Figures
References
-
- Eggermont A., Sileni V.C., Grob J.-J., Dummer R., Wolchok J.D., Schmidt H., Hamid O., Robert C., Ascierto P.A., Richards J.M., et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. - DOI - PubMed
-
- Maio M., Lewis K., Demidov L., Mandalà M., Bondarenko I., Ascierto P.A., Herbert C., Mackiewicz A., Rutkowski P., Guminski A., et al. Adjuvant vemurafenib in resected, BRAF V600 mutation-positive melanoma (BRIM8): A randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018;19:510–520. doi: 10.1016/S1470-2045(18)30106-2. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials