Concentrations of Essential Trace Metals in the Brain of Animal Species-A Comparative Study
- PMID: 32709155
- PMCID: PMC7407190
- DOI: 10.3390/brainsci10070460
Concentrations of Essential Trace Metals in the Brain of Animal Species-A Comparative Study
Abstract
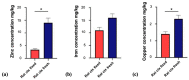
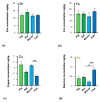
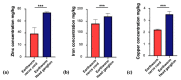
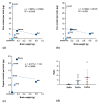
The essential trace metals iron, zinc, and copper have a significant physiological role in healthy brain development and function. Especially zinc is important for neurogenesis, synaptogenesis, synaptic transmission and plasticity, and neurite outgrowth. Given the key role of trace metals in many cellular processes, it is important to maintain adequate levels in the brain. However, the physiological concentration of trace metals, and in particular zinc, in the human and animal brain is not well described so far. For example, little is known about the trace metal content of the brain of animals outside the class of mammals. Here, we report the concentration of iron, zinc, and copper in fresh brain tissue of different model-species of the phyla Chordata (vertebrates (mammals, fish)), Annelida, Arthropoda (insects), and Mollusca (snails), using inductively coupled plasma mass-spectrometry (ICP-MS). Our results show that the trace metals are present in the nervous system of all species and that significant differences can be detected between species of different phyla. We further show that a region-specific distribution of metals within the nervous system already exists in earthworms, hinting at a tightly controlled metal distribution. In line with this, the trace metal content of the brain of different species does not simply correlate with brain size. We conclude that although the functional consequences of the controlled metal homeostasis within the brain of many species remains elusive, trace metal biology may not only play an important role in the nervous system of mammals but across the whole animal kingdom.
Keywords: CNS; ICP-MS; biometals; copper; earthworm; herring; iron; locust; mouse; pig; rat; selenium; snail; zinc.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- World Health Organization . Trace Elements in Human Nutrition and Health. World Health Organization; Geneva, Switzerland: 1996.
LinkOut - more resources
Full Text Sources
Research Materials

