Catabolism and interactions of uncultured organisms shaped by eco-thermodynamics in methanogenic bioprocesses
- PMID: 32709258
- PMCID: PMC7382037
- DOI: 10.1186/s40168-020-00885-y
Catabolism and interactions of uncultured organisms shaped by eco-thermodynamics in methanogenic bioprocesses
Abstract
Background: Current understanding of the carbon cycle in methanogenic environments involves trophic interactions such as interspecies H2 transfer between organotrophs and methanogens. However, many metabolic processes are thermodynamically sensitive to H2 accumulation and can be inhibited by H2 produced from co-occurring metabolisms. Strategies for driving thermodynamically competing metabolisms in methanogenic environments remain unexplored.
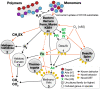
Results: To uncover how anaerobes combat this H2 conflict in situ, we employ metagenomics and metatranscriptomics to revisit a model ecosystem that has inspired many foundational discoveries in anaerobic ecology-methanogenic bioreactors. Through analysis of 17 anaerobic digesters, we recovered 1343 high-quality metagenome-assembled genomes and corresponding gene expression profiles for uncultured lineages spanning 66 phyla and reconstructed their metabolic capacities. We discovered that diverse uncultured populations can drive H2-sensitive metabolisms through (i) metabolic coupling with concurrent H2-tolerant catabolism, (ii) forgoing H2 generation in favor of interspecies transfer of formate and electrons (cytochrome- and pili-mediated) to avoid thermodynamic conflict, and (iii) integration of low-concentration O2 metabolism as an ancillary thermodynamics-enhancing electron sink. Archaeal populations support these processes through unique methanogenic metabolisms-highly favorable H2 oxidation driven by methyl-reducing methanogenesis and tripartite uptake of formate, electrons, and acetate.
Conclusion: Integration of omics and eco-thermodynamics revealed overlooked behavior and interactions of uncultured organisms, including coupling favorable and unfavorable metabolisms, shifting from H2 to formate transfer, respiring low-concentration O2, performing direct interspecies electron transfer, and interacting with high H2-affinity methanogenesis. These findings shed light on how microorganisms overcome a critical obstacle in methanogenic carbon cycles we had hitherto disregarded and provide foundational insight into anaerobic microbial ecology. Video Abstract.
Keywords: Catabolism; Eco-thermodynamics; Interactions; Methanogenic bioprocesses; Uncultured organisms.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Buswell AM. Anaerobic fermentations. State of Illinois Department of Registration and Education. 1936;Bulletin No. 32.
-
- Speece RE. Anaerobic biotechnology for industrial wastewaters. Nashville, TN: Archae Press; 1996. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

