Podocyte Integrin- β3 and Activated Protein C Coordinately Restrict RhoA Signaling and Ameliorate Diabetic Nephropathy
- PMID: 32709711
- PMCID: PMC7460917
- DOI: 10.1681/ASN.2019111163
Podocyte Integrin- β3 and Activated Protein C Coordinately Restrict RhoA Signaling and Ameliorate Diabetic Nephropathy
Abstract
Background: Diabetic nephropathy (dNP), now the leading cause of ESKD, lacks efficient therapies. Coagulation protease-dependent signaling modulates dNP, in part via the G protein-coupled, protease-activated receptors (PARs). Specifically, the cytoprotective protease-activated protein C (aPC) protects from dNP, but the mechanisms are not clear.
Methods: A combination of in vitro approaches and mouse models evaluated the role of aPC-integrin interaction and related signaling in dNP.
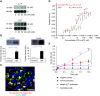
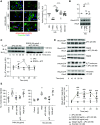
Results: The zymogen protein C and aPC bind to podocyte integrin-β3, a subunit of integrin-αvβ3. Deficiency of this integrin impairs thrombin-mediated generation of aPC on podocytes. The interaction of aPC with integrin-αvβ3 induces transient binding of integrin-β3 with G α13 and controls PAR-dependent RhoA signaling in podocytes. Binding of aPC to integrin-β3via its RGD sequence is required for the temporal restriction of RhoA signaling in podocytes. In podocytes lacking integrin-β3, aPC induces sustained RhoA activation, mimicking the effect of thrombin. In vivo, overexpression of wild-type aPC suppresses pathologic renal RhoA activation and protects against dNP. Disrupting the aPC-integrin-β3 interaction by specifically deleting podocyte integrin-β3 or by abolishing aPC's integrin-binding RGD sequence enhances RhoA signaling in mice with high aPC levels and abolishes aPC's nephroprotective effect. Pharmacologic inhibition of PAR1, the pivotal thrombin receptor, restricts RhoA activation and nephroprotects RGE-aPChigh and wild-type mice.Conclusions aPC-integrin-αvβ3 acts as a rheostat, controlling PAR1-dependent RhoA activation in podocytes in diabetic nephropathy. These results identify integrin-αvβ3 as an essential coreceptor for aPC that is required for nephroprotective aPC-PAR signaling in dNP.
Keywords: RhoA signaling; activated protein C; coagulation proteases; diabetic nephropathy; integrin αvβ3.
Copyright © 2020 by the American Society of Nephrology.
Figures





Similar articles
-
Cytoprotective signaling by activated protein C requires protease-activated receptor-3 in podocytes.Blood. 2012 Jan 19;119(3):874-83. doi: 10.1182/blood-2011-07-365973. Epub 2011 Nov 23. Blood. 2012. PMID: 22117049 Free PMC article.
-
Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis.Nat Med. 2007 Nov;13(11):1349-58. doi: 10.1038/nm1667. Epub 2007 Nov 4. Nat Med. 2007. PMID: 17982464
-
Activated protein C promotes protease-activated receptor-1 cytoprotective signaling through β-arrestin and dishevelled-2 scaffolds.Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):E1372-80. doi: 10.1073/pnas.1112482108. Epub 2011 Nov 21. Proc Natl Acad Sci U S A. 2011. PMID: 22106258 Free PMC article.
-
The cytoprotective protein C pathway.Blood. 2007 Apr 15;109(8):3161-72. doi: 10.1182/blood-2006-09-003004. Epub 2006 Nov 16. Blood. 2007. PMID: 17110453 Review.
-
Activated protein C, protease activated receptor 1, and neuroprotection.Blood. 2018 Jul 12;132(2):159-169. doi: 10.1182/blood-2018-02-769026. Epub 2018 Jun 4. Blood. 2018. PMID: 29866816 Free PMC article. Review.
Cited by
-
The integrin repertoire drives YAP-dependent epithelial:stromal interactions during injury of the kidney glomerulus.Nat Commun. 2025 Apr 8;16(1):3322. doi: 10.1038/s41467-025-58567-y. Nat Commun. 2025. PMID: 40199893 Free PMC article.
-
Protease-Activated Receptor 1-Mediated Damage of Podocytes in Diabetic Nephropathy.Diabetes. 2023 Dec 1;72(12):1795-1808. doi: 10.2337/db23-0032. Diabetes. 2023. PMID: 37722138 Free PMC article.
-
Urolithin A Ameliorates the TGF Beta-Dependent Impairment of Podocytes Exposed to High Glucose.J Pers Med. 2024 Aug 28;14(9):914. doi: 10.3390/jpm14090914. J Pers Med. 2024. PMID: 39338168 Free PMC article.
-
The role and mechanism of butyrate in the prevention and treatment of diabetic kidney disease.Front Microbiol. 2022 Aug 9;13:961536. doi: 10.3389/fmicb.2022.961536. eCollection 2022. Front Microbiol. 2022. PMID: 36016798 Free PMC article. Review.
-
Hypercoagulability Impairs Plaque Stability in Diabetes-Induced Atherosclerosis.Nutrients. 2022 May 10;14(10):1991. doi: 10.3390/nu14101991. Nutrients. 2022. PMID: 35631132 Free PMC article.
References
-
- Fouli GE, Gnudi L: The future: experimental therapies for renal disease in diabetes. Nephron 143: 3–7, 2019. - PubMed
-
- Gaede P, Tarnow L, Vedel P, Parving HH, Pedersen O: Remission to normoalbuminuria during multifactorial treatment preserves kidney function in patients with type 2 diabetes and microalbuminuria. Nephrol Dial Transplant 19: 2784–2788, 2004. - PubMed
-
- Bock F, Shahzad K, Vergnolle N, Isermann B: Activated protein C based therapeutic strategies in chronic diseases. Thromb Haemost 111: 610–617, 2014. - PubMed
-
- Isermann B: Homeostatic effects of coagulation protease-dependent signaling and protease activated receptors. J Thromb Haemost 15: 1273–1284, 2017. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

