Baseline metabolic tumor volume as a strong predictive and prognostic biomarker in patients with non-small cell lung cancer treated with PD1 inhibitors: a prospective study
- PMID: 32709713
- PMCID: PMC7380842
- DOI: 10.1136/jitc-2020-000645
Baseline metabolic tumor volume as a strong predictive and prognostic biomarker in patients with non-small cell lung cancer treated with PD1 inhibitors: a prospective study
Abstract
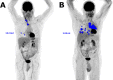
Background: Reliable predictive and prognostic markers are still lacking for patients treated with programmed death receptor 1 (PD1) inhibitors for non-small cell lung cancer (NSCLC). The purpose of this study was to investigate the prognostic and predictive values of different baseline metabolic parameters, including metabolic tumor volume (MTV), from 18F-fluorodeoxyglucose positron emission tomography-computed tomography (18F-FDG PET/CT) scans in patients with NSCLC treated with PD1 inhibitors.
Methods: Maximum and peak standardized uptake values, MTV and total lesion glycolysis (TLG), as well as clinical and biological parameters, were recorded in 75 prospectively included patients with NSCLC treated with PD1 inhibitors. Associations between these parameters and overall survival (OS) were evaluated as well as their accuracy to predict early treatment discontinuation (ETD).
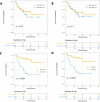
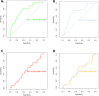
Results: A high MTV and a high TLG were significantly associated with a lower OS (p<0.001). The median OS in patients with MTV above the median (36.5 cm3) was 10.5 months (95% CI: 6.2 to upper limit: unreached), while the median OS in patients with MTV below the median was not reached. Patients with no prior chemotherapy had a poorer OS than patients who had received prior systemic treatment (p=0.04). MTV and TLG could reliably predict ETD (area under the receiver operating characteristic curve=0.76, 95% CI: 0.65 to 0.87 and 0.72, 95% CI: 0.62 to 0.84, respectively).
Conclusion: MTV is a strong prognostic and predictive factor in patients with NSCLC treated with PD1 inhibitors and can be easily determined from routine 18F-FDG PET/CT scans. MTV, could help to personalize immunotherapy and be used to stratify patients in future clinical studies.
Keywords: biomarkers, tumor; immunotherapy; lung neoplasms; tumor biomarkers.
© Author(s) (or their employer(s)) 2020. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MI reports personal fees from AstraZeneca, Bristol-Myers Squibb, Roche, Boehringer-Ingelheim, and Merck & Co outside the submitted work.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
