Absence of cGAS-mediated type I IFN responses in HIV-1-infected T cells
- PMID: 32709741
- PMCID: PMC7431009
- DOI: 10.1073/pnas.2002481117
Absence of cGAS-mediated type I IFN responses in HIV-1-infected T cells
Abstract
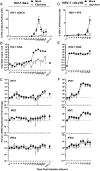
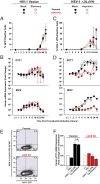
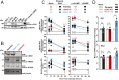
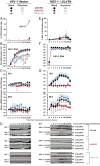
The DNA sensor cGAS catalyzes the production of the cyclic dinucleotide cGAMP, resulting in type I interferon responses. We addressed the functionality of cGAS-mediated DNA sensing in human and murine T cells. Activated primary CD4+ T cells expressed cGAS and responded to plasmid DNA by upregulation of ISGs and release of bioactive interferon. In mouse T cells, cGAS KO ablated sensing of plasmid DNA, and TREX1 KO enabled cells to sense short immunostimulatory DNA. Expression of IFIT1 and MX2 was downregulated and upregulated in cGAS KO and TREX1 KO T cell lines, respectively, compared to parental cells. Despite their intact cGAS sensing pathway, human CD4+ T cells failed to mount a reverse transcriptase (RT) inhibitor-sensitive immune response following HIV-1 infection. In contrast, infection of human T cells with HSV-1 that is functionally deficient for the cGAS antagonist pUL41 (HSV-1ΔUL41N) resulted in a cGAS-dependent type I interferon response. In accordance with our results in primary CD4+ T cells, plasmid challenge or HSV-1ΔUL41N inoculation of T cell lines provoked an entirely cGAS-dependent type I interferon response, including IRF3 phosphorylation and expression of ISGs. In contrast, no RT-dependent interferon response was detected following transduction of T cell lines with VSV-G-pseudotyped lentiviral or gammaretroviral particles. Together, T cells are capable to raise a cGAS-dependent cell-intrinsic response to both plasmid DNA challenge or inoculation with HSV-1ΔUL41N. However, HIV-1 infection does not appear to trigger cGAS-mediated sensing of viral DNA in T cells, possibly by revealing viral DNA of insufficient quantity, length, and/or accessibility to cGAS.
Keywords: HIV-1; HSV-1; T cells; cGAS; innate sensing.
Copyright © 2020 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: W.B. is an employee of IFM Therapeutics.
Figures



References
-
- Ablasser A., et al. , TREX1 deficiency triggers cell-autonomous immunity in a cGAS-dependent manner. J. Immunol. 192, 5993–5997 (2014). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

