Thinner biological tissues induce leaflet flutter in aortic heart valve replacements
- PMID: 32709744
- PMCID: PMC7431095
- DOI: 10.1073/pnas.2002821117
Thinner biological tissues induce leaflet flutter in aortic heart valve replacements
Abstract
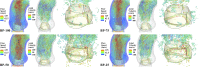
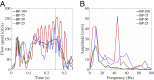
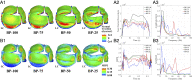
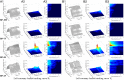
Valvular heart disease has recently become an increasing public health concern due to the high prevalence of valve degeneration in aging populations. For patients with severely impacted aortic valves that require replacement, catheter-based bioprosthetic valve deployment offers a minimally invasive treatment option that eliminates many of the risks associated with surgical valve replacement. Although recent percutaneous device advancements have incorporated thinner, more flexible biological tissues to streamline safer deployment through catheters, the impact of such tissues in the complex, mechanically demanding, and highly dynamic valvular system remains poorly understood. The present work utilized a validated computational fluid-structure interaction approach to isolate the behavior of thinner, more compliant aortic valve tissues in a physiologically realistic system. This computational study identified and quantified significant leaflet flutter induced by the use of thinner tissues that initiated blood flow disturbances and oscillatory leaflet strains. The aortic flow and valvular dynamics associated with these thinner valvular tissues have not been previously identified and provide essential information that can significantly advance fundamental knowledge about the cardiac system and support future medical device innovation. Considering the risks associated with such observed flutter phenomena, including blood damage and accelerated leaflet deterioration, this study demonstrates the potentially serious impact of introducing thinner, more flexible tissues into the cardiac system.
Keywords: fluid–structure interaction; heart valves; immersogeometric analysis; leaflet flutter; thin biological tissues.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Nkomo V. T., et al. , Burden of valvular heart diseases: A population-based study. Lancet 368, 1005–1011 (2006.) - PubMed
-
- Vesely I., The evolution of bioprosthetic heart valve design and its impact on durability. Cardiovasc. Pathol. 12, 277–286 (2003). - PubMed
-
- Ishihara T., Ferrans V. J., Boyce S. W., Jones M., Roberts W. C., Structure and classification of cuspal tears and perforations in porcine bioprosthetic cardiac valves implanted in patients. Am. J. Cardiol. 48, 665–678 (1981). - PubMed
-
- Pomar J. L., Bosch X., Chaitman B. R., Pelletier C., Grondin C. M.. Late tears in leaflets of porcine bioprostheses in adults. Ann. Thorac. Surg. 37, 78–83 (1984). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

