Endogenous toxic metabolites and implications in cancer therapy
- PMID: 32709924
- PMCID: PMC7452860
- DOI: 10.1038/s41388-020-01395-9
Endogenous toxic metabolites and implications in cancer therapy
Abstract
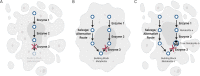
It is well recognized that many metabolic enzymes play essential roles in cancer cells in producing building blocks such as nucleotides, which are required in greater amounts due to their increased proliferation. On the other hand, the significance of enzymes in preventing the accumulation of their substrates is less recognized. Here, we outline the evidence and underlying mechanisms for how many metabolites normally produced in cells are highly toxic, such as metabolites containing reactive groups (e.g., methylglyoxal, 4-hydroxynonenal, and glutaconyl-CoA), or metabolites that act as competitive analogs against other metabolites (e.g., deoxyuridine triphosphate and l-2-hydroxyglutarate). Thus, if a metabolic pathway contains a toxic intermediate, then we may be able to induce accumulation and poison a cancer cell by targeting the downstream enzyme. Furthermore, this poisoning may be cancer cell selective if this pathway is overactive in a cancer cell relative to a nontransformed cell. We describe this concept as illustrated in selenocysteine metabolism and other pathways and discuss future directions in exploiting toxic metabolites to kill cancer cells.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

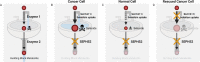
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123:309–14. - PubMed
-
- Yang L, Venneti S, Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017;19:163–94. - PubMed
-
- Swinnen JV, Brusselmans K, Verhoeven G. Increased lipogenesis in cancer cells: new players, novel targets. Curr Opin Clin Nutr Metab Care. 2006;9:358–65. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

