High-throughput production of cheap mineral-based two-dimensional electrocatalysts for high-current-density hydrogen evolution
- PMID: 32709937
- PMCID: PMC7381654
- DOI: 10.1038/s41467-020-17121-8
High-throughput production of cheap mineral-based two-dimensional electrocatalysts for high-current-density hydrogen evolution
Abstract
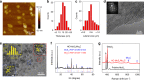
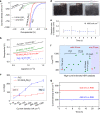
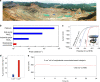
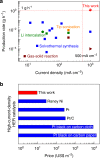
The high-throughput scalable production of cheap, efficient and durable electrocatalysts that work well at high current densities demanded by industry is a great challenge for the large-scale implementation of electrochemical technologies. Here we report the production of a two-dimensional molybdenum disulfide-based ink-type electrocatalyst by a scalable exfoliation technique followed by a thermal treatment. The catalyst delivers a high current density of 1000 mA cm-2 at an overpotential of 412 mV for the hydrogen evolution. Using the same method, we produce a cheap mineral-based catalyst possessing excellent performance for high-current-density hydrogen evolution. Noteworthy, production rate of this catalyst is one to two orders of magnitude higher than those previously reported, and price of the mineral is five orders of magnitude lower than commercial Pt electrocatalysts. These advantages indicate the huge potentials of this method and of mineral-based cheap and abundant natural resources as catalysts in the electrochemical industry.
Conflict of interest statement
Patents related to this research have been filed by Tsinghua-Berkeley Shenzhen Institute, Tsinghua University. The University’s policy is to share financial rewards from the exploitation of patents with the inventors.
Figures

References
-
- Kibsgaard J, Chorkendorff I. Considerations for the scaling-up of water splitting catalysts. Nat. Energy. 2019;4:430–433. doi: 10.1038/s41560-019-0407-1. - DOI
-
- Zhang B, et al. Interface engineering: The Ni(OH)2/MoS2 heterostructure for highly efficient alkaline hydrogen evolution. Nano Energy. 2017;37:74–80. doi: 10.1016/j.nanoen.2017.05.011. - DOI
-
- National Minerals Information Center. Available from: https://www.usgs.gov/centers/nmic/commodity-statistics-and-information.
Grants and funding
LinkOut - more resources
Full Text Sources

