Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing
- PMID: 32709961
- PMCID: PMC7948516
- DOI: 10.1038/s41573-020-0072-x
Brain energy rescue: an emerging therapeutic concept for neurodegenerative disorders of ageing
Abstract
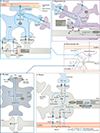
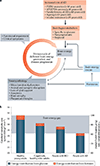
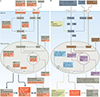
The brain requires a continuous supply of energy in the form of ATP, most of which is produced from glucose by oxidative phosphorylation in mitochondria, complemented by aerobic glycolysis in the cytoplasm. When glucose levels are limited, ketone bodies generated in the liver and lactate derived from exercising skeletal muscle can also become important energy substrates for the brain. In neurodegenerative disorders of ageing, brain glucose metabolism deteriorates in a progressive, region-specific and disease-specific manner - a problem that is best characterized in Alzheimer disease, where it begins presymptomatically. This Review discusses the status and prospects of therapeutic strategies for countering neurodegenerative disorders of ageing by improving, preserving or rescuing brain energetics. The approaches described include restoring oxidative phosphorylation and glycolysis, increasing insulin sensitivity, correcting mitochondrial dysfunction, ketone-based interventions, acting via hormones that modulate cerebral energetics, RNA therapeutics and complementary multimodal lifestyle changes.
Figures
References
-
- Aldana BI Microglia-specific metabolic changes in neurodegeneration. J. Mol. Biol. 431, 1830–1842 (2019). - PubMed
-
- Zilberter Y & Zilberter M The vicious circle of hypometabolism in neurodegenerative diseases: ways and mechanisms of metabolic correction. J. Neurosci. Res. 95, 2217–2235 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
- R15 AG050292/AG/NIA NIH HHS/United States
- R01 NS107265/NS/NINDS NIH HHS/United States
- R37 AG053589/AG/NIA NIH HHS/United States
- RF1 AG062135/AG/NIA NIH HHS/United States
- RF1 AG055549/AG/NIA NIH HHS/United States
- R01 AG060733/AG/NIA NIH HHS/United States
- MC_U105663142/MRC_/Medical Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- P01 AG026572/AG/NIA NIH HHS/United States
- MC_UU_00015/3/MRC_/Medical Research Council/United Kingdom
- R21 AG064479/AG/NIA NIH HHS/United States
- P30 AG035982/AG/NIA NIH HHS/United States
- UH3 NS113776/NS/NINDS NIH HHS/United States
- RF1 AG059093/AG/NIA NIH HHS/United States
- R01 AG057931/AG/NIA NIH HHS/United States
- R01 AG061194/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

