Long-term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: A pooled analysis of four randomized trials
- PMID: 32710498
- PMCID: PMC7520354
- DOI: 10.1002/cam4.3298
Long-term overall survival and toxicities of ABVD vs BEACOPP in advanced Hodgkin lymphoma: A pooled analysis of four randomized trials
Abstract
Purpose: We explored the potential overall survival (OS) benefit of bleomycin, etoposide, doxorubicin (Adriamycin), cyclophosphamide, vincristine (Oncovin), procarbazine, and prednisone (BEACOPP) over doxorubicin (Adriamycin), bleomycin, vinblastine, and dacarbazine (ABVD) in a pooled analysis of four randomized trials.
Patients and methods: Primary objective was to evaluate the OS impact of BEACOPP using individual patient data. Secondary objectives were progression-free survival (PFS), secondary cancers, and use of autologous stem cell transplantation (ASCT).
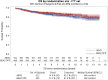
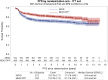
Results: About 1227 patients were included. The 7-year OS was 84.3% (95% CI 80.8-87.2) for ABVD vs 87.7% (95% CI 84.5-90.2) for BEACOPP. Two follow-up periods were identified based on survival curves and hazard ratio (HR) over time. For the first 18 months, there was no difference. For the second period of ≥18 months, ABVD patients had a higher death risk (HRABVD vs BEACOPP = 1.59; 95% CI 1.09-2.33). A Cox model stratified by trial and evaluating the effect of treatment and International Prognostic Index (IPI) score as fixed effects showed that both were statistically significant (treatment, P = .0185; IPI score, P = .0107). The 7-year PFS was 71.1% (95% CI 67.1-74.6) for ABVD vs 81.1% (95% CI 77.5-84.2) for BEACOPP (P < .001). After ABVD, 25 secondary cancers (4.0%) were reported with no myelodysplasia (MDS)/acute myeloid leukemia (AML) compared to 36 (6.5%) after BEACOPP, which included 13 patients with MDS/AML. Following ABVD, 86 patients (13.8%) received ASCT vs 39 (6.4%) for BEACOPP.
Conclusions: This analysis showed a slight improvement in OS for BEACOPP and confirmed a PFS benefit. Frontline use of BEACOPP instead of ABVD increased secondary leukemia incidence but halved the requirement for ASCT.
Keywords: ABVD; BEACOPP; Hodgkin lymphoma; overall survival; progression-free survival; secondary cancers.
© 2020 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors do not have any relevant conflict of interest to disclose.
Figures
References
-
- Canellos GP, Anderson JR, Propert KJ, et al. Chemotherapy of advanced Hodgkin's disease with MOPP, ABVD, or MOPP alternating with ABVD. N Engl J Med. 1992;327:1478‐1484. - PubMed
-
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased‐dose BEACOPP chemotherapy compared with COPP‐ABVD for advanced Hodgkin's disease. New Engl J Med. 2003;348:2386‐2395. - PubMed
-
- Federico M, Luminari S, Iannitto E, et al. ABVD compared with BEACOPP compared with CEC for the initial treatment of patients with advanced Hodgkin's lymphoma: results from the HD2000 Gruppo Italiano per lo Studio dei Linfomi Trial. J Clin Oncol. 2009;27:805‐811. - PubMed
-
- Viviani S, Zinzani PL, Rambaldi A, et al. ABVD versus BEACOPP for Hodgkin's lymphoma when high‐dose salvage is planned. N Engl J Med. 2011;365:203‐212. - PubMed
-
- Mounier N, Brice P, Bologna S, et al. ABVD (8 cycles) versus BEACOPP (4 escalated cycles ≥ 4 baseline): final results in stage III‐IV low‐risk Hodgkin lymphoma (IPS 0–2) of the LYSA H34 randomized trial. Ann Oncol. 2014;25:1622‐1628. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

