Rationale, design, and baseline characteristics of the Salt Substitute in India Study (SSiIS): The protocol for a double-blinded, randomized-controlled trial
- PMID: 32710677
- PMCID: PMC8029753
- DOI: 10.1111/jch.13947
Rationale, design, and baseline characteristics of the Salt Substitute in India Study (SSiIS): The protocol for a double-blinded, randomized-controlled trial
Abstract
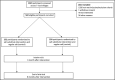
Reduced-sodium, added-potassium salt substitutes have favorable effects on blood pressure, but have not been tested in India. The Salt Substitute in India Study (SSiIS) is a double-blinded, randomized-controlled trial designed to investigate the effects of reduced-sodium, added-potassium salt substitution to replace usual cooking salt use and blood pressure (BP) among hypertensive patients in rural India. The primary objective is to assess effects on systolic blood pressure at 3 months. The secondary objectives are to determine effects on diastolic blood pressure, urinary sodium, and potassium levels, and to determine acceptability of the intervention. Eligible individuals received usual salt (100% sodium chloride) or salt substitute (70% sodium chloride and 30% potassium chloride) to replace all salt required for cooking and seasoning in the household. A total of 502 participants aged ≥18 years with a history of hypertension were successfully recruited and randomized in a 1:1 ratio to intervention or control, between November 2019 and January 2020. Mean blood pressure at baseline was 133.5/83.6 mm Hg and 96% were using one or more blood pressure-lowering medications. The overall mean average 24-hour urinary sodium excretion was 2825 (SD, 1166) mg/L, which corresponds to a urinary salt excretion of 10.4 g/d. Baseline findings suggest sodium intake in this population significantly exceeds World Health Organization recommendations. The SSiIS trial has successfully recruited participants and is well placed to determine whether salt substitution is an effective means of lowering blood pressure for rural Indian patients with hypertension.
Keywords: India; blood pressure; hypertension; salt substitute.
©2020 Wiley Periodicals LLC.
Conflict of interest statement
The study protocol received ethics approval from the George Institute Ethics Committee (Project Number 09‐2019). Written informed consent was obtained from all study participants and their household members. An external Independent Safety Monitor who is an expert in clinical trials has been appointed. The monitor will be provided with data about all Serious Adverse Events as they accrue during the study and will be asked to advise the investigators if at any point during the course of the study there is any reason for concern. The Independent Safety Monitor will be supported by a trial statistician who will provide the data required from the relevant participant and perform additional analyses as might be requested by the Independent Safety Monitor.
Figures
Comment in
-
Maximizing the potential of the Salt Substitute in India Study.J Clin Hypertens (Greenwich). 2021 Jan;23(1):197-198. doi: 10.1111/jch.14109. Epub 2020 Nov 21. J Clin Hypertens (Greenwich). 2021. PMID: 33222393 Free PMC article. No abstract available.
References
-
- Patel V, Chatterji S, Chisholm D, et al. Chronic diseases and injuries in India. Lancet (London, England). 2011;377(9763):413–28. - PubMed
-
- Midha T, Idris MZ, Saran RK, Srivastav AK, Singh SK. Prevalence and determinants of hypertension in the urban and rural population of a north Indian district. East African journal of public health. 2009;6(3):268–73. - PubMed
-
- R. k. Suma, T. r. Mayamol, Divakaran Binoo, Karunakaran Usha, A. K. Jayasree. Hypertension: prevalence, awareness, treatment and control in a rural area of North Kerala, India. Int J Commun Med Public Health. 2017;4(10):3561–3567.
-
- Mozaffarian D, Fahimi S, Singh GM, et al. Global sodium consumption and death from cardiovascular causes. N Engl J Med. 2014;371(7):624–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical