In-silico human electro-mechanical ventricular modelling and simulation for drug-induced pro-arrhythmia and inotropic risk assessment
- PMID: 32710902
- PMCID: PMC7848595
- DOI: 10.1016/j.pbiomolbio.2020.06.007
In-silico human electro-mechanical ventricular modelling and simulation for drug-induced pro-arrhythmia and inotropic risk assessment
Abstract
Human-based computational modelling and simulation are powerful tools to accelerate the mechanistic understanding of cardiac patho-physiology, and to develop and evaluate therapeutic interventions. The aim of this study is to calibrate and evaluate human ventricular electro-mechanical models for investigations on the effect of the electro-mechanical coupling and pharmacological action on human ventricular electrophysiology, calcium dynamics, and active contraction. The most recent models of human ventricular electrophysiology, excitation-contraction coupling, and active contraction were integrated, and the coupled models were calibrated using human experimental data. Simulations were then conducted using the coupled models to quantify the effects of electro-mechanical coupling and drug exposure on electrophysiology and force generation in virtual human ventricular cardiomyocytes and tissue. The resulting calibrated human electro-mechanical models yielded active tension, action potential, and calcium transient metrics that are in agreement with experiments for endocardial, epicardial, and mid-myocardial human samples. Simulation results correctly predicted the inotropic response of different multichannel action reference compounds and demonstrated that the electro-mechanical coupling improves the robustness of repolarisation under drug exposure compared to electrophysiology-only models. They also generated additional evidence to explain the partial mismatch between in-silico and in-vitro experiments on drug-induced electrophysiology changes. The human calibrated and evaluated modelling and simulation framework constructed in this study opens new avenues for future investigations into the complex interplay between the electrical and mechanical cardiac substrates, its modulation by pharmacological action, and its translation to tissue and organ models of cardiac patho-physiology.
Keywords: Computational modelling; Computer simulation; Drug safety; Human cardiac contraction; Human ventricular action potential; In-silico drug trials.
Copyright © 2020 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest None.
Figures



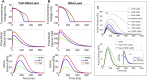



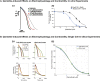
Similar articles
-
Development, calibration, and validation of a novel human ventricular myocyte model in health, disease, and drug block.Elife. 2019 Dec 24;8:e48890. doi: 10.7554/eLife.48890. Elife. 2019. PMID: 31868580 Free PMC article.
-
A mathematical model of hiPSC cardiomyocytes electromechanics.Physiol Rep. 2021 Nov;9(22):e15124. doi: 10.14814/phy2.15124. Physiol Rep. 2021. PMID: 34825519 Free PMC article.
-
Multi-scale approaches for the simulation of cardiac electrophysiology: I - Sub-cellular and stochastic calcium dynamics from cell to organ.Methods. 2021 Jan;185:49-59. doi: 10.1016/j.ymeth.2020.02.011. Epub 2020 Feb 29. Methods. 2021. PMID: 32126258 Review.
-
Mechanisms of transmurally varying myocyte electromechanics in an integrated computational model.Philos Trans A Math Phys Eng Sci. 2008 Sep 28;366(1879):3361-80. doi: 10.1098/rsta.2008.0088. Philos Trans A Math Phys Eng Sci. 2008. PMID: 18593662 Free PMC article.
-
Comprehensive in vitro cardiac safety assessment using human stem cell technology: Overview of CSAHi HEART initiative.J Pharmacol Toxicol Methods. 2017 Jan-Feb;83:42-54. doi: 10.1016/j.vascn.2016.09.004. Epub 2016 Sep 17. J Pharmacol Toxicol Methods. 2017. PMID: 27646297 Review.
Cited by
-
Inflammation as a Risk Factor in Cardiotoxicity: An Important Consideration for Screening During Drug Development.Front Pharmacol. 2021 Apr 19;12:598549. doi: 10.3389/fphar.2021.598549. eCollection 2021. Front Pharmacol. 2021. PMID: 33953668 Free PMC article.
-
A systematic review of cardiac in-silico clinical trials.Prog Biomed Eng (Bristol). 2023 Jul 1;5(3):032004. doi: 10.1088/2516-1091/acdc71. Epub 2023 Jun 22. Prog Biomed Eng (Bristol). 2023. PMID: 37360227 Free PMC article. Review.
-
Preclinical Models of Cardiac Disease: A Comprehensive Overview for Clinical Scientists.Cardiovasc Eng Technol. 2024 Apr;15(2):232-249. doi: 10.1007/s13239-023-00707-w. Epub 2024 Jan 16. Cardiovasc Eng Technol. 2024. PMID: 38228811 Free PMC article. Review.
-
An Electromechanical Model-Based Study on the Dosage Effects of Ranolazine in Treating Failing HCM Cardiomyocyte.Cell Mol Bioeng. 2025 Feb 21;18(2):137-162. doi: 10.1007/s12195-025-00842-5. eCollection 2025 Apr. Cell Mol Bioeng. 2025. PMID: 40290110
-
Mechanism based therapies enable personalised treatment of hypertrophic cardiomyopathy.Sci Rep. 2022 Dec 28;12(1):22501. doi: 10.1038/s41598-022-26889-2. Sci Rep. 2022. PMID: 36577774 Free PMC article.
References
-
- Augustin C.M., Neic A., Liebmann M., Prassl A.J., Niederer S.A., Haase G., Plank G. Anatomically accurate high resolution modeling of human whole heart electromechanics: a strongly scalable algebraic multigrid solver method for nonlinear deformation. J. Comput. Phys. 2016;305:622–646. doi: 10.1016/j.jcp.2015.10.045. - DOI - PMC - PubMed
-
- Britton O.J., Abi-Gerges N., Page G., Ghetti A., Miller P.E., Rodriguez B. Quantitative comparison of effects of Dofetilide, sotalol, quinidine, and verapamil between human ex vivo trabeculae and in silico ventricular models incorporating inter-individual action potential variability. Front. Physiol. 2017;8 doi: 10.3389/fphys.2017.00597. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

