COVID-19 and multiple sclerosis: A description of two cases on alemtuzumab
- PMID: 32711297
- PMCID: PMC7366111
- DOI: 10.1016/j.msard.2020.102402
COVID-19 and multiple sclerosis: A description of two cases on alemtuzumab
Abstract
Background: Alemtuzumab is a treatment for highly active multiple sclerosis (MS). Immunosuppression is considered a risk factor for SARS-CoV-2 infection and there is still lack of evidence to guide MS practice.
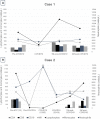
Methods/results: We describe the clinical and immunological evolution of two MS patients under alemtuzumab treatment who were affected by COVID-19, one of them only one week after receiving her last dose, and both recovered without sequelae.
Conclusion: In selected patients (young, without comorbidities, and with high activity), MS itself could be more dangerous than COVID-19, so we should consider continuing MS treatment as previously planned, including alemtuzumab.
Keywords: Alemtuzumab; COVID-19; Immunosuppression; Multiple sclerosis; SARS-CoV-2.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declared the following potential conflicts of interest: EFD and JGG have received research support, compensation for participating on advisory boards, lecture fees and/or travel support from: Almirall, Bayer, Genzyme-Sanofi, Novartis, Roche and Teva declare no potential conflicts of interest. JGGG has received research support, compensation for participating on advisory boards, lecture fees and/or travel support from: AbbVie, Bayer, Novartis, Angellini and Allergan. MP and CMRS have received support to attend congresses and conferences from Merck. TS has received compensation for participating in scientific advisory boards of Amgen Inc and Boehringer Ingelheim, and funding for travel or speaker honoraria of Bayer Pharmaceuticals and Daiichi-Sankyo. None of these companies are involved in the choice of content, writing or decision to publish this manuscript.
Figures
References
-
- Carandini T., Pietroboni A.M., Sacchi L., De Riz M.A., Pozzato M., Arighi A., Fumagalli G.G., Martinelli Boneschi F., Galimberti D., Scarpini E. Alemtuzumab in multiple sclerosis during the COVID-19 pandemic: a mild uncomplicated infection despite intense immunosuppression. Mult. Scler. 2020 doi: 10.1177/1352458520926459. 1352458520926459. - DOI - PubMed
-
- Costa-Frossard França L., Moreno Torres I., Meca Lallana V., García Domínguez J.M., en representación del grupo de est, en representación del grupo de est Documento EMCAM (Esclerosis Múltiple Comunidad de Madrid) para manejo de pacientes con Esclerosis Múltiple durante la pandemia SARS-CoV2. Rev. Neurol. 2020;70:1. doi: 10.33588/rn.7009.2020155. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

