Digital technologies for an improved management of respiratory allergic diseases: 10 years of clinical studies using an online platform for patients and physicians
- PMID: 32711557
- PMCID: PMC7382563
- DOI: 10.1186/s13052-020-00870-z
Digital technologies for an improved management of respiratory allergic diseases: 10 years of clinical studies using an online platform for patients and physicians
Abstract
Background: Digital health technologies carry the great potential of assisting physicians in making well-informed diagnostic and therapeutic decisions. In allergy care, electronic clinical diaries have been recently used to prospectively collect patient data and improve diagnostic precision.
Objective: This review summarizes the clinical and scientific experience we gathered over 10 years of using a digital platform for patients suffering from seasonal allergic rhinitis.
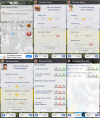
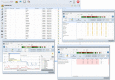
Methods: The mobile application and back-office of AllergyMonitor (TPS software production, Rome, Italy) enable patients to record their daily allergy symptoms as well as drug and immunotherapy intake plus possible side effects in a customizable way. The results can be accessed by the patient and attending physician as concise reports via a smartphone or computer. This technology has been used in several clinical studies and routine practice since 2009.
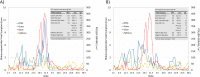
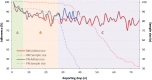
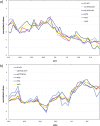
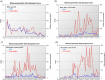
Results: Our studies showed that A) the etiological diagnosis of SAR may be supported by matching prospectively registered symptoms with pollen counts; B) it is possible to perform a short-term prediction of SAR-symptoms at individual level; C) the adherence to daily symptom monitoring can remain high (> 80%) throughout several weeks when prescribed and thoroughly explained by the treating doctor; D) the use of mobile technology can improve adherence to symptomatic drugs as well as allergen-specific immunotherapy and E) the choice of the correct symptom-severity-score is critical at patient level, but not at group level.
Conclusion: The studies and clinical practice based on the use of AllergyMonitor have proven the reliability and positive impact of a digital platform including an electronic diary (eDiary) on the diagnostic precision of SAR in poly-sensitized patients as well as patient adherence to both, drug therapy and allergen immunotherapy.
Keywords: Digital; E-diary; Mobile health; Pollen; Precision medicine.
Conflict of interest statement
Dr. Tripodi reports personal fees from TPS Production srl, during the conduct of the study; In addition, Dr. Tripodi has a patent 102,017,000,106,570 issued to TPS Production srl. Simone Pelosi reports personal fees from TPS Production srl. P.M. Matricardi is funded by the Deutsche Forschungsgemeinschaft (DFG; grant number MA 4740/2–1), is a consultant for HYCOR Biomedical, Euroimmun, Thermo Fisher Scientific (TFS), has received research funding from HYCOR Biomedical, Euroimmun, reagents for research from Thermofisher; and speaker’s fees from Euroimmun, Thermo Fisher Scientific, Stallergenes-Greer, HAL Allergy. All other authors declare that they have no conflict of interest.
Figures



References
-
- World Health Organization, https://www.who.int/tb/areas-of-work/digital-health/faq/en/. Last accessed on 19 Jan 2020).
-
- Free C, Phillips G, Galli L, Watson L, Felix L, Edwards P, et al. The effectiveness of mobileHealth technology-based health behaviour change or disease management interventions for health care consumers: a systematic review. PLoS Med. 2013;10:e1001362. doi: 10.1371/journal.pmed.1001362. - DOI - PMC - PubMed
-
- World Health Organization . mHealth, Use of appropriate digital technologies for public health. Seventy-first World Health Assembly. A71/20. 2018.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

