Effect of food on the pharmacokinetics of omeprazole, pantoprazole and rabeprazole
- PMID: 32711578
- PMCID: PMC7382816
- DOI: 10.1186/s40360-020-00433-2
Effect of food on the pharmacokinetics of omeprazole, pantoprazole and rabeprazole
Abstract
Background: The pharmacokinetics of proton pump inhibitors (PPIs) may be affected by food intake. We aimed to evaluate the effect of food on the pharmacokinetics of omeprazole, rabeprazole, and pantoprazole.
Setting: The study population comprised 186 healthy volunteers participating in 6 bioequivalence clinical trials.
Method: Subjects were evaluated to determine the effect of a high-fat breakfast on the pharmacokinetics of omeprazole (n = 36), rabeprazole (n = 69), and pantoprazole (n = 81).
Main outcome measure: Drug plasma concentrations were measured using high-performance liquid chromatography coupled to mass spectrometry.
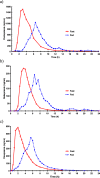
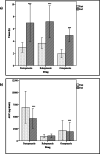
Results: Food affected the pharmacokinetics of omeprazole (increased Tmax and decreased AUC and Cmax), pantoprazole (increased Tmax and decreased AUC), and rabeprazole (increased Tmax, Cmax and half-life). Food increased variability in Tmax for all 3 drugs, delaying absorption around 3 to 4 h and until 20 h in some subjects.
Conclusion: As food delays the absorption of PPIs and increases their variability, it would be better to administer these drugs under fasting conditions.
Trial registration: European Union Drug Regulating Authorities Clinical Trials Database: EudraCT : 2004-003863-59 (registration date 05/MAR/2004), EudraCT 2006-001162-17 (registration date 17-MAR-2006), EudraCT: 2007-002489-37 (registration date 12-JUN-2007), EudraCT: 2007-002490-31 (registration date 12-JUN-2007), EudraCT: 2010-024029-19 (registration date 23-NOV-2010).
Keywords: Food; Omeprazole; Pantoprazole; Pharmacokinetics; Proton pump inhibitors; Rabeprazole.
Conflict of interest statement
F.A.S. and D.O. have been consultants or investigators in clinical trials sponsored by the following pharmaceutical companies: Abbott, Alter, Chemo, Cinfa, FAES, Farmalíder, Ferrer, GlaxoSmithKline, Galenicum, Gilead, Italfarmaco, Janssen-Cilag, Kern, Normon, Novartis, Servier, Silverpharma, Teva and Zambon. The remaining authors declare no competing interests.
Figures
Similar articles
-
Evaluation of the relationship between polymorphisms in CYP2C19 and the pharmacokinetics of omeprazole, pantoprazole and rabeprazole.Pharmacogenomics. 2014;15(15):1893-901. doi: 10.2217/pgs.14.141. Pharmacogenomics. 2014. PMID: 25495411
-
Effect of CYP2C19*2 and *17 mutations on pharmacodynamics and kinetics of proton pump inhibitors in Caucasians.Br J Clin Pharmacol. 2008 May;65(5):752-60. doi: 10.1111/j.1365-2125.2007.03094.x. Epub 2008 Jan 30. Br J Clin Pharmacol. 2008. PMID: 18241283 Free PMC article. Clinical Trial.
-
Stereoselective disposition of proton pump inhibitors.Clin Drug Investig. 2008;28(5):263-79. doi: 10.2165/00044011-200828050-00001. Clin Drug Investig. 2008. PMID: 18407713 Review.
-
Pharmacokinetics of proton pump inhibitors in children.Clin Pharmacokinet. 2005;44(5):441-66. doi: 10.2165/00003088-200544050-00001. Clin Pharmacokinet. 2005. PMID: 15871633 Review.
-
Comparative in vitro assessment of CYP2C19 inhibition by ilaprazole and conventional proton pump inhibitors using a high throughput fluorometric assay.Sci Rep. 2025 May 25;15(1):18158. doi: 10.1038/s41598-025-02872-5. Sci Rep. 2025. PMID: 40415025 Free PMC article.
Cited by
-
Evaluation of the cytochrome P450 2C19 and 3A4 inhibition potential of the complement factor 5a receptor 1 antagonist ACT-1014-6470 in vitro and in vivo.Clin Transl Sci. 2023 Jul;16(7):1220-1231. doi: 10.1111/cts.13525. Epub 2023 Apr 26. Clin Transl Sci. 2023. PMID: 37042126 Free PMC article.
-
Knowledge, Attitudes, and Practices of Community Pharmacists Regarding Proton Pump Inhibitor (PPI) Use: A Cross-Sectional Study.Healthcare (Basel). 2025 Jul 2;13(13):1588. doi: 10.3390/healthcare13131588. Healthcare (Basel). 2025. PMID: 40648613 Free PMC article.
-
Survey-Based Analysis of Current Trends for Prescribing Gastrointestinal Protectants among Small-Animal General Practitioners in Portugal.Vet Sci. 2021 Apr 23;8(5):70. doi: 10.3390/vetsci8050070. Vet Sci. 2021. PMID: 33922570 Free PMC article.
-
Effect of Food and Dosing Regimen on Safety and Efficacy of Proton Pump Inhibitors Therapy-A Literature Review.Int J Environ Res Public Health. 2021 Mar 29;18(7):3527. doi: 10.3390/ijerph18073527. Int J Environ Res Public Health. 2021. PMID: 33805341 Free PMC article. Review.
-
The Influence of CYP3A4 Genetic Polymorphism and Proton Pump Inhibitors on Osimertinib Metabolism.Front Pharmacol. 2022 Mar 10;13:794931. doi: 10.3389/fphar.2022.794931. eCollection 2022. Front Pharmacol. 2022. PMID: 35359868 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

