Can Activation of NRF2 Be a Strategy against COVID-19?
- PMID: 32711925
- PMCID: PMC7359808
- DOI: 10.1016/j.tips.2020.07.003
Can Activation of NRF2 Be a Strategy against COVID-19?
Abstract
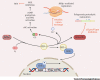
Acute respiratory distress syndrome (ARDS) caused by SARS-CoV-2 is largely the result of a dysregulated host response, followed by damage to alveolar cells and lung fibrosis. Exacerbated proinflammatory cytokines release (cytokine storm) and loss of T lymphocytes (leukopenia) characterize the most aggressive presentation. We propose that a multifaceted anti-inflammatory strategy based on pharmacological activation of nuclear factor erythroid 2 p45-related factor 2 (NRF2) can be deployed against the virus. The strategy provides robust cytoprotection by restoring redox and protein homeostasis, promoting resolution of inflammation, and facilitating repair. NRF2 activators such as sulforaphane and bardoxolone methyl are already in clinical trials. The safety and efficacy information of these modulators in humans, together with their well-documented cytoprotective and anti-inflammatory effects in preclinical models, highlight the potential of this armamentarium for deployment to the battlefield against COVID-19.
Keywords: KEAP1; SARS-CoV-2; anti-inflammatory ARDS; bardoxolone methyl; sulforaphane.
Copyright © 2020 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Galani I.E., Andreakos E. Neutrophils in viral infections: current concepts and caveats. J. Leukoc. Biol. 2015;98:557–564. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

