Progression of Fatty Liver Disease in Children Receiving Standard of Care Lifestyle Advice
- PMID: 32712103
- PMCID: PMC7680281
- DOI: 10.1053/j.gastro.2020.07.034
Progression of Fatty Liver Disease in Children Receiving Standard of Care Lifestyle Advice
Abstract
Background & aims: Nonalcoholic fatty liver disease (NAFLD) is the most common pediatric chronic liver disease. Little is known about outcomes in recognized youth.
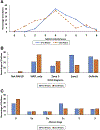
Methods: We compared paired liver biopsies from 122 of 139 children with NAFLD (74% male; 64% white; 71% Hispanic; mean age, 13 ± 3 years; age range, 8-17 years) who received placebo and standard of care lifestyle advice in 2 double-blind, randomized clinical trials within the nonalcoholic steatohepatitis (NASH) clinical research network from 2005 through 2015. We analyzed histologic changes with respect to baseline and longitudinal change in clinical variables using regression analysis.
Results: At enrollment, 31% of the children had definite NASH, 34% had borderline zone 1 NASH, 13% had borderline zone 3 NASH, and 21% had fatty liver but not NASH. Over a mean period of 1.6 ± 0.4 years, borderline or definite NASH resolved in 29% of the children, whereas 18% of the children with fatty liver or borderline NASH developed definite NASH. Fibrosis improved in 34% of the children but worsened in 23%. Any progression to definite NASH and/or in fibrosis was associated with adolescent age, and higher waist circumference, levels of alanine or aspartate aminotransferase, total and low-density lipoprotein cholesterol at baseline (<0.05), and over follow-up time, with increasing level of alanine aminotransferase, hemoglobin A1C (P<.05), gamma-glutamyl transferase and development of type 2 diabetes (P<.01). Increasing level of gamma-glutamyl transferase was also associated with reduced odds of any improvement (P = .003).
Conclusions: One-third of children with NAFLD enrolled in placebo groups of clinical trials had histologic features of progression within 2 years, in association with increasing obesity and serum levels of aminotransferases and loss of glucose homeostasis.
Keywords: ALT; Cirrhosis; Histology; Natural History.
Copyright © 2020 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of Interest
These authors disclose the following: Cynthia Behling is a consultant to ICON and Covance. Elizabeth Brunt is a consultant to Perspectum Diagnostics and Histoindex. Ajay Jain is a consultant to Alexion. Joel Lavine is a consultant for Surrozen, Intercept, and Novo Nordisk. He received grant support from Genfit. Tamir Miloh is a consultant to Alexion. Jean Molleston has received research support from Gilead, Abbvie, Albireo, and Shire/Mirum. Karen Murray is a consultant to Gilead and Albireo. Philip Rosenthal has received research support from Gilead, Abbvie, BMS, Roche/Genentech, Retrophin, Merck, Albireo, Mirum, and Arrowhead and has served as a consultant for Albireo, Audentes, Retrophin, Gilead, Abbvie, Dicerna, Arrowhead and Mirum. Arun Sanyal is president of Sanyal Biotechnology and has stock options in Genfit, Akarna, Tiziana, Indalo, Durect Inversago, and Galmed. He has served as a consultant to Astra Zeneca, Nitto Denko, Conatus, Nimbus, Salix, Tobira, Takeda, Jannsen, Gilead, Terns, Birdrock, Merck, Valeant, Boehringer-Ingelheim, Bristol Myers Squibb, Lilly, Hemoshear, Zafgen, Novartis, Novo Nordisk, Pfizer, Exhalenz and Genfit. He has been an unpaid consultant to Intercept, Echosens, Immuron, Galectin, Fractyl, Syntlogic, Affimune, Chemomab, Zydus, Nordic Bioscience, Albireo, Prosciento, Surrozen. His institution has received grant support from Gilead, Salix, Tobira, Bristol Myers, Shire, Intercept, Merck, Astra Zeneca, Malinckrodt, Cumberland and Novartis. He receives royalties from Elsevier and UptoDate. Ann Scheimann has received research support from Millendo Therapeutics, Insys Therapeutics, Albireo, and serves on a Data Safety Monitoring Board for Levo Therapeutics. Jeffrey Schwimmer has received grants from Galmed, Genfit, and Intercept. Miriam Vos has received grants from Gemphire, Resonance Health, and Shire, and is a consultant for Boehringer Ingelheim, Bristol Myers Squibb, Immuron, Intercept, Mallinckrodt, Novo Nordisk, Target Pharmasolutions, and AMRA. Stavra Xanthakos has received grants from Axcella Health and Target PharmaSolutions. She receives royalties from UptoDate. The remaining authors disclose no conflicts.
Figures


References
-
- Schwimmer JB, Deutsch R, Kahen T, et al. Prevalence of fatty liver in children and adolescents. Pediatrics 2006; 118:1388–1393. - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357. - PubMed
-
- Yeh MM, Brunt EM. Pathological features of fatty liver disease. Gastroenterology 2014;147:754–764. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK061731/DK/NIDDK NIH HHS/United States
- U01 DK061718/DK/NIDDK NIH HHS/United States
- P30 DK078392/DK/NIDDK NIH HHS/United States
- UL1 TR000006/TR/NCATS NIH HHS/United States
- UL1 TR001422/TR/NCATS NIH HHS/United States
- U01 DK061728/DK/NIDDK NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- UL1 TR000448/TR/NCATS NIH HHS/United States
- U01 DK061734/DK/NIDDK NIH HHS/United States
- UL1 TR000004/TR/NCATS NIH HHS/United States
- UL1 TR002345/TR/NCATS NIH HHS/United States
- U01 DK061737/DK/NIDDK NIH HHS/United States
- U01 DK061713/DK/NIDDK NIH HHS/United States
- U01 DK061732/DK/NIDDK NIH HHS/United States
- UL1 TR000150/TR/NCATS NIH HHS/United States
- U01 DK061730/DK/NIDDK NIH HHS/United States
- U24 DK061730/DK/NIDDK NIH HHS/United States
- U01 DK061738/DK/NIDDK NIH HHS/United States
- UL1 TR000424/TR/NCATS NIH HHS/United States
- UL1 TR000040/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
- UL1 TR000423/TR/NCATS NIH HHS/United States
- UL1 TR000100/TR/NCATS NIH HHS/United States

