Inter and Intracellular mitochondrial trafficking in health and disease
- PMID: 32712108
- PMCID: PMC7484258
- DOI: 10.1016/j.arr.2020.101128
Inter and Intracellular mitochondrial trafficking in health and disease
Abstract
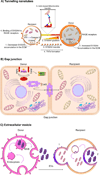
Neurons and glia maintain central nervous system (CNS) homeostasis through diverse mechanisms of intra- and intercellular signaling. Some of these interactions include the exchange of soluble factors between cells via direct cell-to-cell contact for both short and long-distance transfer of biological materials. Transcellular transfer of mitochondria has emerged as a key example of this communication. This transcellular transfer of mitochondria are dynamically involved in the cellular and tissue response to CNS injury and play beneficial roles in recovery. This review highlights recent research addressing the cause and effect of intra- and intercellular mitochondrial transfer with a specific focus on the future of mitochondrial transplantation therapy. We believe that mitochondrial transfer plays a crucial role during bioenergetic crisis/deficit, but the quality, quantity and mode of mitochondrial transfer determines the protective capacity for the receiving cells. Mitochondrial transplantation is a new treatment paradigm and will overcome the major bottleneck of traditional approach of correcting mitochondria-related disorders.
Keywords: Extracellular vesicles; Kinesin; Miro; Mitochondrial extrusion; Mitochondrial transplantation; TRAK; Tunneling nanotubes.
Copyright © 2020 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of Interest
No potential conflict of interest was reported by the authors
Figures


References
-
- Abounit S, Zurzolo C, 2012. Wiring through tunneling nanotubes--from electrical signals to organelle transfer. J Cell Sci 125, 1089–1098. - PubMed
-
- Ahmad T, Mukherjee S, Pattnaik B, Kumar M, Singh S, Kumar M, Rehman R, Tiwari BK, Jha KA, Barhanpurkar AP, Wani MR, Roy SS, Mabalirajan U, Ghosh B, Agrawal A, 2014. Miro1 regulates intercellular mitochondrial transport & enhances mesenchymal stem cell rescue efficacy. EMBO J 33, 994–1010. - PMC - PubMed
-
- Al Amir Dache Z, Otandault A, Tanos R, Pastor B, Meddeb R, Sanchez C, Arena G, Lasorsa L, Bennett A, Grange T, El Messaoudi S, Mazard T, Prevostel C, Thierry AR, 2020. Blood contains circulating cell-free respiratory competent mitochondria. FASEB J 34, 3616–3630. - PubMed
-
- Araki T, 1977. Freezing injury in mitochondrial membranes. II. Degradation of phospholipid in rabbit liver mitochondria during freezing and storage at low temperatures. Cryobiology 14, 151–159. - PubMed
-
- Aspenstrom P, Ruusala A, Pacholsky D, 2007. Taking Rho GTPases to the next level: the cellular functions of atypical Rho GTPases. Exp Cell Res 313, 3673–3679. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

